Sulfur, due to its diverse oxidation states, is widespread in biologically active molecules and serves as a fundamental element in drug discovery. Among sulfur-containing motifs, sulfinamides with an S(IV) stereogenic center have become increasingly important scaffolds because of their distinctive physicochemical properties and pharmacokinetic features, and they can function as ligands or chiral auxiliaries in transition-metal catalysis as well as precursors to S(VI) functionalities (e.g., sulfoximines and sulfonimidamides). Conventional syntheses of sulfinamides from thiols mainly rely on nonradical, multistep routes, including prefunctionalization of thiols into thioethers, sulfonyl chlorides, or sulfinates/sulfinate esters followed by reactions with amines, or nitrogen electrophilic reactions between sulfinate-derived anion precursors and amines. These approaches are commonly limited by laborious substrate preparation, poor stability of sulfur intermediates, harsh conditions, and narrow substrate scope. Direct synthesis of sulfinamides from thiols remains underdeveloped, largely because oxidation is difficult to control. Under electrochemical conditions, thiols and amines can generate radicals; direct S–N bond formation via sulfur/nitrogen radical cross-coupling could complement existing methods, but key challenges such as radical homodimerization and selective oxidation control still need to be addressed.
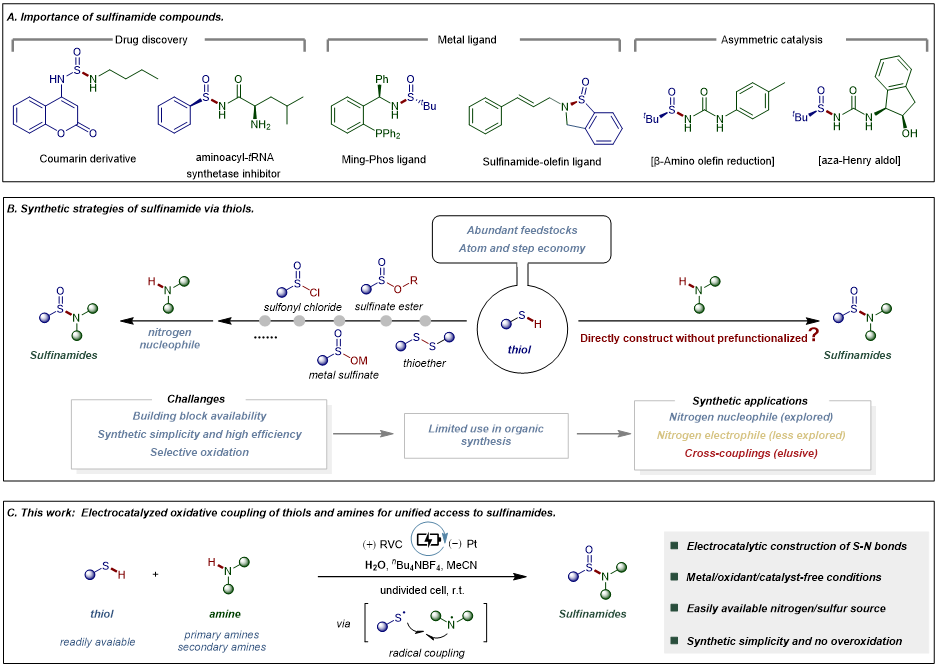
Fig. 1. Breaking the nonradical limitation for the synthesis of sulfinamides by electrooxidation.
Recently, Prof. Qingmin Wang’s group at Nankai University reported an efficient electrocatalytic strategy for the synthesis of sulfinamides via an intermolecular oxidative cross-coupling between readily available aryl thiols and aliphatic amines. Importantly, the generated nitrogen radicals selectively react with sulfur radicals to form S–N bonds rather than undergoing homodimerization. The key advantages of this method include: (1) a radical-based S–N bond-forming strategy that complements conventional nonradical approaches; (2) broad substrate scope with excellent functional-group tolerance; and (3) potential for scale-up as well as applicability to late-stage functionalization of complex molecules. Mechanistic studies indicate that four consecutive anodic oxidations play a central role in radical generation, S–N bond formation, and further oxidation of intermediates. Relevant achievements were published in Science Advances, DOI: 10.1126/sciadv.aeb6913.