Polyketides are a class of natural products with diverse structures and biological activities such as antibacterial and anticancer properties. These molecules often contain multiple contiguous units including alkenyl, methyl, and alkoxy groups, exhibiting complex stereochemical features. These functional groups recur in polyketide frameworks in various oxidized or reduced forms, constituting the core structures of numerous pharmaceuticals and clinical candidates. Therefore, the development of efficient and highly stereoselective methods for the synthesis of polyketide structures is of great significance. Traditional polyketide synthesis generally relies on two-component coupling reactions (such as asymmetric aldol, allylation, or propargylation reactions) as well as multi-step functional group manipulations. Although these methods can precisely control the stereochemistry of molecules, they often require stoichiometric organometallic reagents. In recent years, transition metal-catalyzed asymmetric reductive coupling reactions have overcome the aforementioned limitations to a certain extent. Nevertheless, such linear or convergent strategies still involve numerous steps, leading to reduced overall yields and thereby limiting the diversity-oriented synthesis of compounds. Thus, the development of new catalytic asymmetric multicomponent synthesis strategies with simplified steps and high efficiency is of important research significance for enriching the synthetic methodology system of polyketides.
In recent years, the research group led by Li-Jun Xiao at Nankai University has long been dedicated to studies on catalytic synthesis based on the oxidative cyclometalation strategy. Focusing on the efficient transformation of alkenes with various unsaturated compounds (such as aldehydes, ketones, and imines) under nickel catalysis, the group has advanced the development of new green synthesis methods. Recently, by developing a chiral spiro ligand-nickel catalyst with dual CH−π interactions, the Li-Jun Xiao group has achieved the asymmetric multicomponent coupling reaction of 1,3-dienes, aldehydes, and boronic acids, providing a novel approach for the highly stereoselective synthesis of polyketide structures (Figure 1). This method exhibits the following prominent advantages: (1) efficiently constructing two carbon−carbon bonds in a single step; (2) precisely forming olefin units with defined geometry; (3) obtaining two adjacent chiral centers with excellent enantioselectivity and diastereoselectivity; (4) enabling efficient access to all stereoisomers, demonstrating outstanding stereocontrol capability; (5) mechanistic studies reveal that the CH−π interactions between the ligand and substrate play a crucial role in stereoselectivity. Compared with traditional two-component coupling reactions, this three-component coupling strategy significantly enhances the atom economy and step efficiency in the synthesis of polyketide structural units, offering a new perspective for the efficient synthesis of polyketide molecules. Relevant achievements were published in J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c17153.
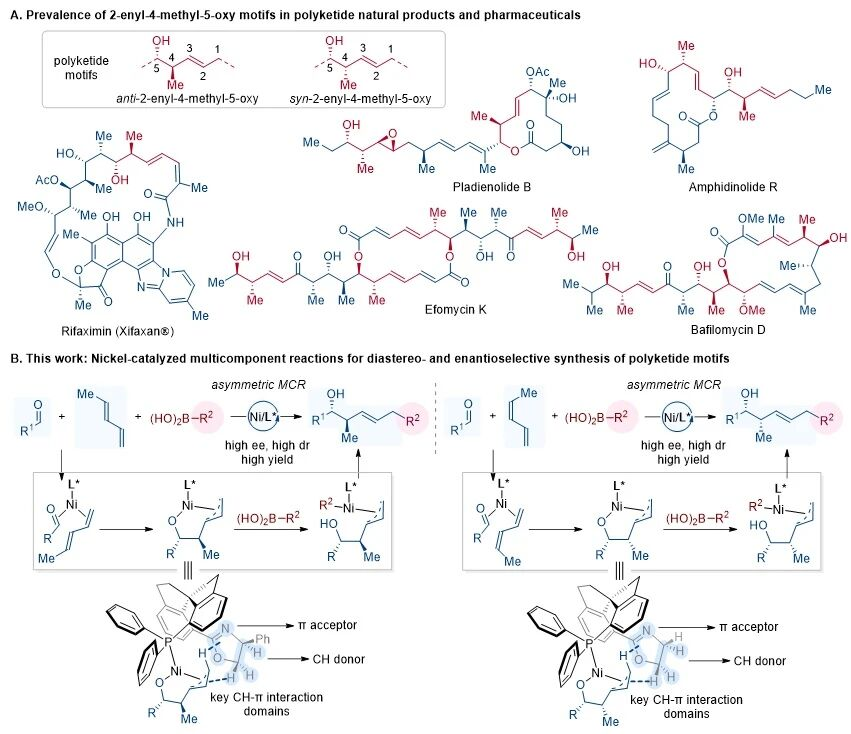
Figure 1. Examples of Relevant Polyketide Structures and Nickel-Catalyzed Asymmetric Multicomponent Coupling Reactions