Trifluoromethoxylated aromatics have emerged as privileged structural motifs in medicinal and agrochemical fields, owing to its unique steric and electronic properties (Figure 1A). Specifically, the orthogonal orientation of the trifluoromethoxy group (OCF3) relative to the aromatic plane enhances binding affinity with biological targets, while its pronounced lipophilicity and electron-withdrawing character contribute to improved metabolic stability of bioactive molecules. Despite the growing importance of trifluoromethoxylated aromatics, their synthesis via direct trifluoromethoxylation of aromatic starting materials remains a formidable task. The inherent thermal instability and weak nucleophilicity of the OCF3 group pose significant synthetic hurdles (Figure 1B).
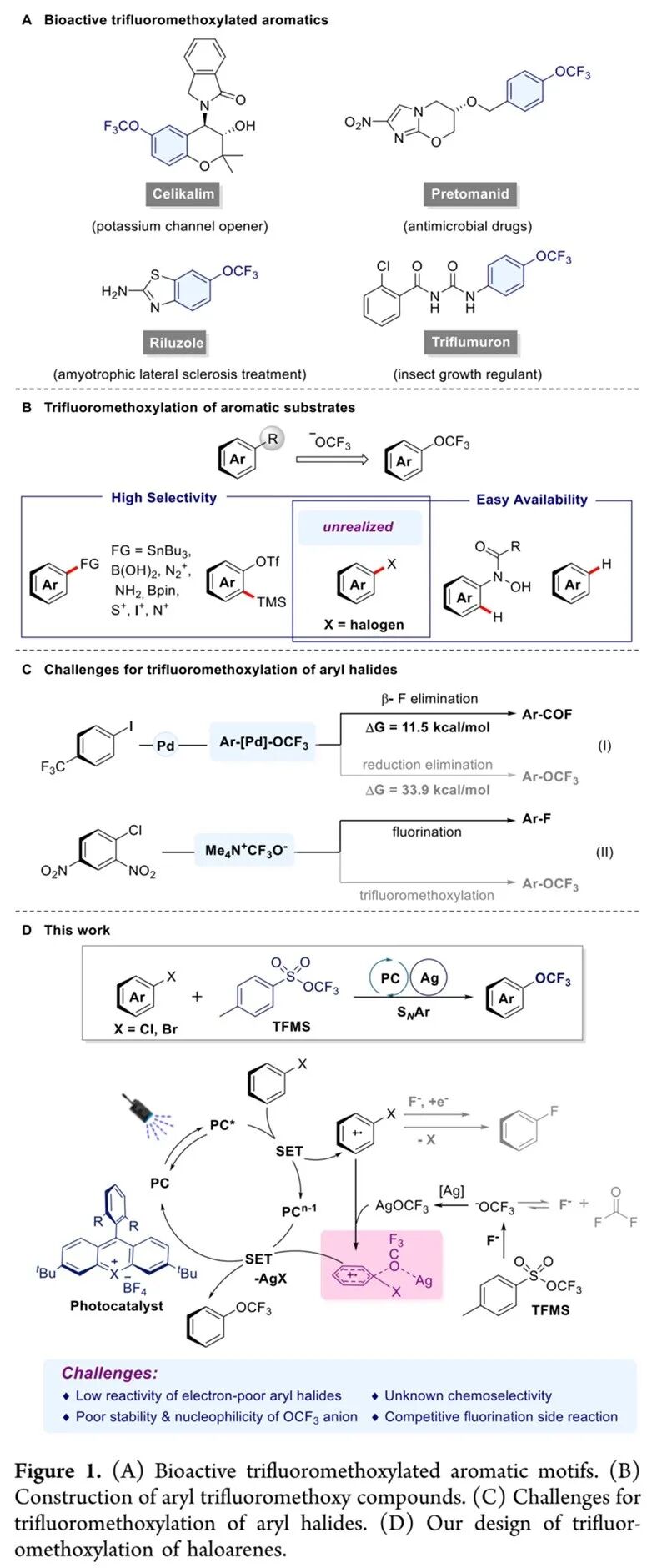
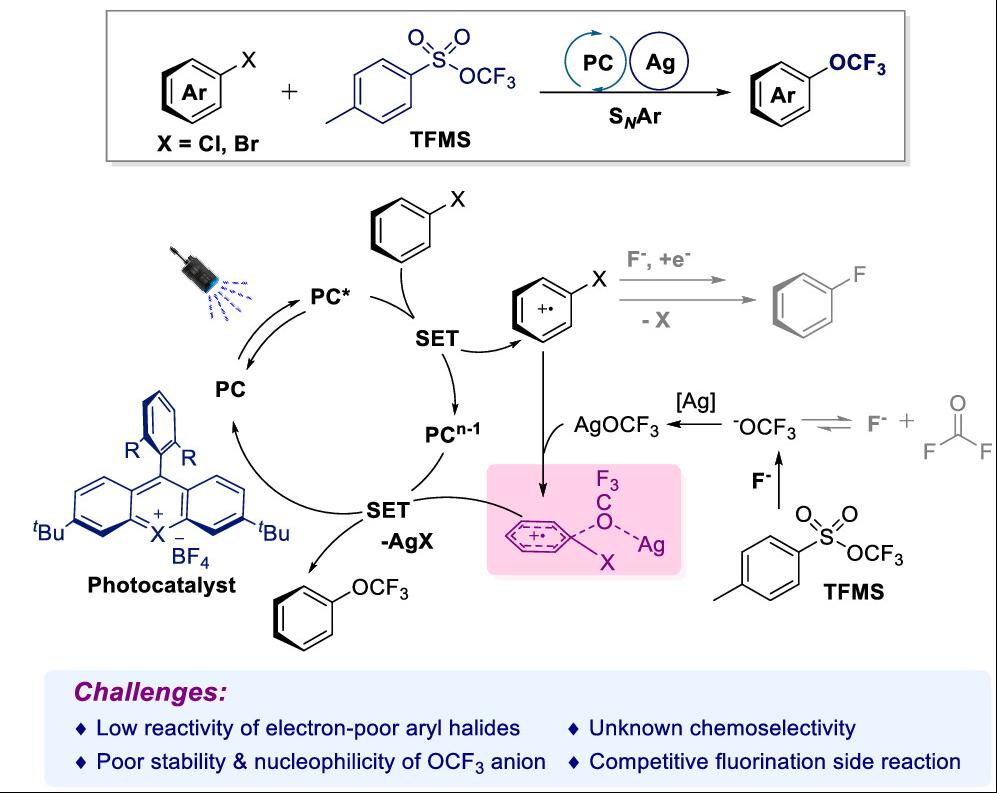
Recently, Pingping Tang’s team, for the first time, has successfully achieved the trifluoromethoxylation of aryl halides via photocatalyzed aryl cationic radical intermediates. By introducing silver salts, challenging inert electron-neutral and electron-deficient aryl halides also participated smoothly in the reaction. As a result, various trifluoromethoxylated aromatics were obtained in up to 95% isolated yields. Further DFT calculations revealed that the presence of silver facilitates the departure of the halogen, with a lower energy barrier, thereby enhancing the reactivity of inert aryl halides. This method also accommodates bioactive aromatic molecules as substrates, indicating further application in medicinal chemistry. Beyond its immediate synthetic utility, the silver-enabled promotion protocol may also inspire other efficient systems for challenging transformations with implications spanning organic synthesis, materials science, and drug discovery. Relevant achievements were published in J. Am. Chem. Soc., 2025, 10.1021/jacs.5c11167.
Read more:
Photocatalyst-Regulated Trifluoromethoxylation of Aryl Halides under Silver Promotion
Jingya Zhou, Longhui Chen, Ziang Miao, Jinjin Li, Yu-Xin Luan,* Li Chen,* and Pingping Tang*
J. Am. Chem. Soc. 2025, 147, 38979−38986, DOI: 10.1021/jacs.5c11167