The selective chemical modification of biomolecules is pivotal for advancing modern biomedical research. While significant progress has been made in coupling strategies targeting highly reactive yet low-abundance thiol groups (e.g., cysteine), developing chemical methods to target more abundant native functional groups—particularly the primary amines of lysine side chains—remains a major challenge. An ideal strategy should maintain high reactivity and precision while being orthogonal to thiol chemistry, enabling multi-site or multiplexed bioconjugation. However, the reactivity of amine groups is constrained by their weak nucleophilicity and protonation under physiological pH, rendering thiol–amine orthogonal labeling persistently elusive.
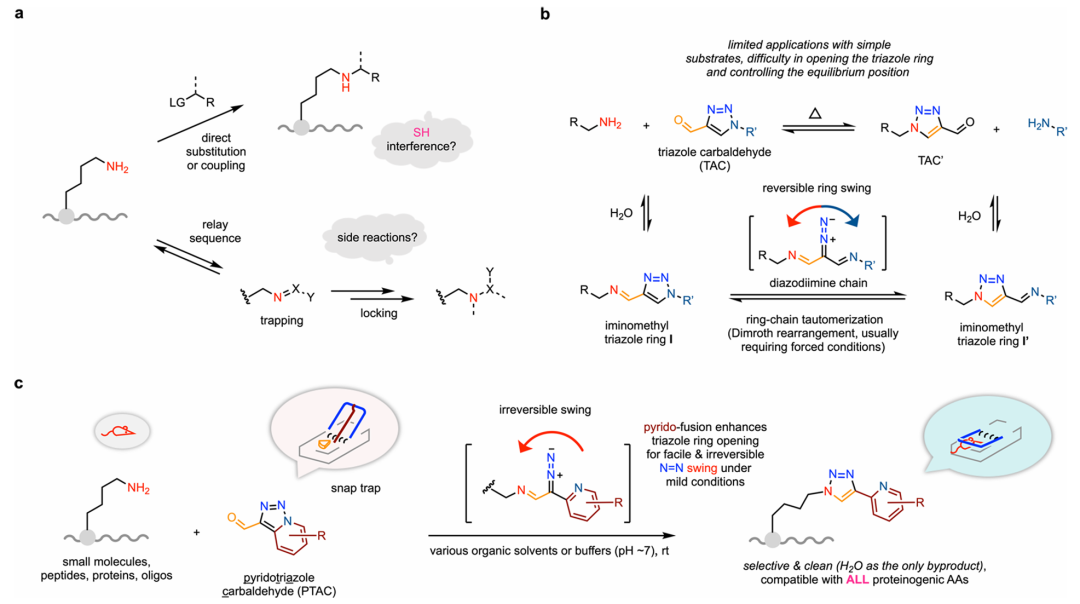
Recently, the teams of Gong Chen (Nankai University) and Hua-Dong Xu (Changzhou University) reported a novel class of pyridotriazole carbaldehyde (PTAC) reagents capable of efficient, selective, and highly clean labeling of primary alkylamines in both small molecules and complex biomolecules under mild, biocompatible conditions. PTACs operate via a unique "snap-trapping" mechanism: the aldehyde bait first forms an imine intermediate with the amine, followed by irreversible capture through tautomerization-mediated triazole ring swinging, releasing water as the sole byproduct. Fusion of a pyridine ring into the triazole core destabilizes the N–N bond, enabling selective activation by amines while ensuring excellent stability and strict thiol orthogonality. PTACs exhibit broad compatibility with native functional groups, achieving unprecedented efficiency in amine labeling. By integrating high reactivity, chemoselectivity, and functional group tolerance, PTAC reagents deliver thiol-level performance in amine-specific bioconjugation, offering both a compelling alternative and valuable complement to established thiol-targeting methods. Relevant achievements were published in J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c15886.