Over the past decade, organic electrochemistry has experienced rapid development, primarily due to its ability to obviate the need for stoichiometric chemical oxidants or reductants in traditional transformations. Consequently, it is recognized as a green alternative to traditional reactions while also providing opportunities to discover new reactivities that leverage the generation of highly reactive and controllable radical species within the electrochemical cell. Through the precise control of redox potentials via applied current, potential, or other operational parameters, this approach can suppress side reactions and prevent the over-reduction of intermediates, thereby enhancing chemoselectivity and efficiency. These inherent advantages have driven the advancement of selective electroreductive transformations. The selective electroreduction of aromatic carboxylic acid derivatives is a significant transformation in organic chemistry, yet achieving both selectivity and generality across a broad range of carboxylic acid derivatives remains challenging.

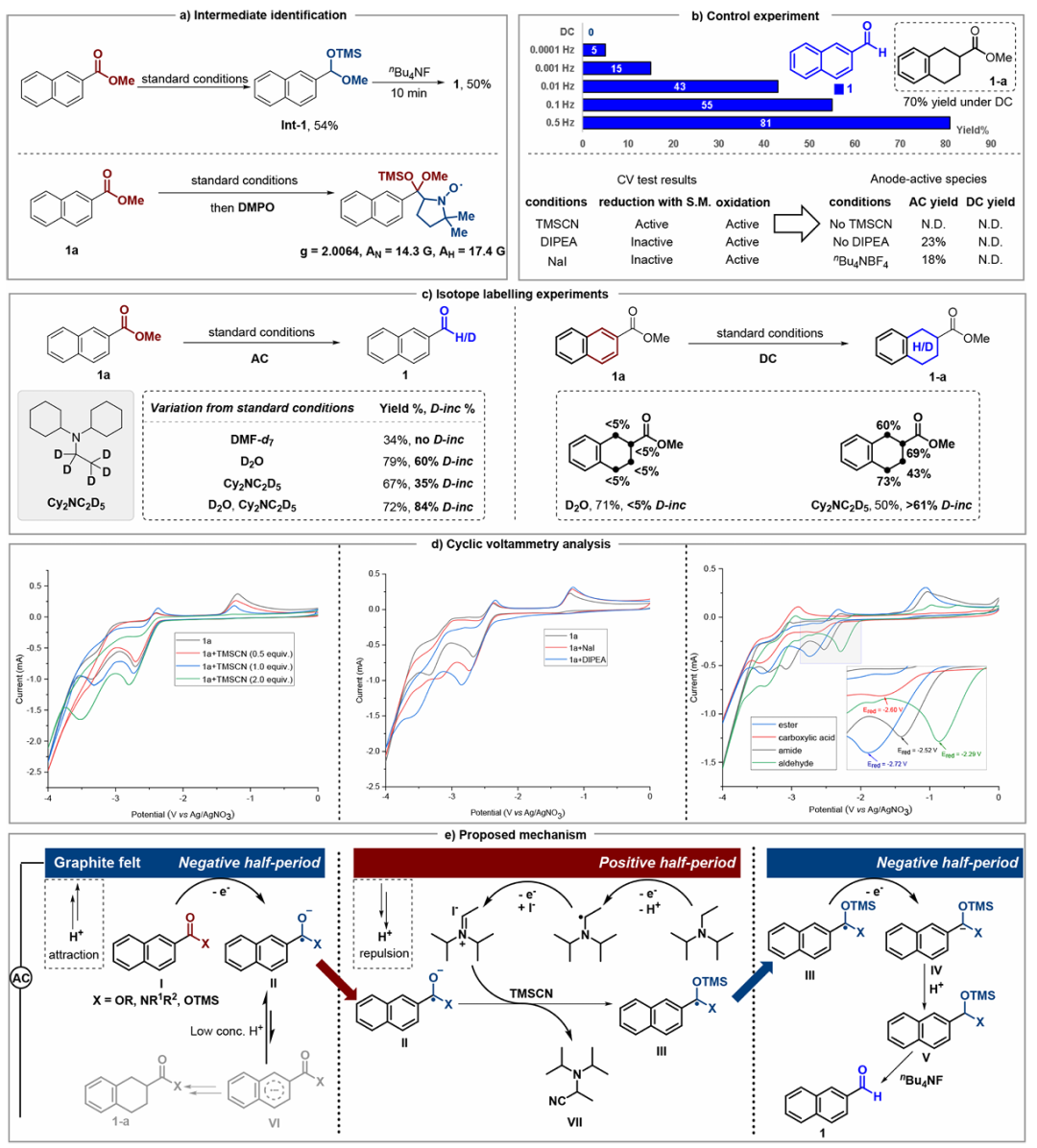
Building upon recent advances in selective electroreduction, the research groups of Qi-Lin Zhou and Youai Qiu recently reported a silicon-assisted alternating current (AC) electroreduction strategy for the selective conversion of diverse aromatic carboxylic acids and their derivatives into aromatic aldehydes. This protocol exhibits high chemoselectivity, broad substrate scope, and excellent functional group tolerance under mild conditions, enabling efficient synthesis of a wide range of aromatic aldehydes. Mechanistic studies suggest the involvement of a ketyl radical intermediate, highlighting the critical role of silicon moieties in promoting the formation of the final aldehyde products. Relevant achievements were published in J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c14069.