The construction of quaternary carbon centers has long been a prominent research focus in organic synthesis, yet poses significant challenges due to substantial steric hindrance and competitive side reactions. Transition-metal-catalyzed C–C coupling reactions represent a direct strategy for constructing these all-carbon quaternary centers. Currently, couplings involving tertiary C(sp3) sites have been extensively investigated, with the majority of reports focusing on reactions with C(sp2) species, whereas couplings with other C(sp3) centers remain relatively rare and predominantly rely on air/moisture-sensitive metallic reagents (e.g., Grignard and zinc reagents) displaying limited functional group tolerance (Fig. 1a). Transition-metal-catalyzed reductive coupling reactions, which circumvent the need for sensitive organometallic reagents, offer a more practical approach for accessing quaternary carbon centers. Nickel catalysts have been shown to mediate reductive couplings between tertiary alkyl halides and allylic esters/benzyl chlorides (Fig. 1b). Recently, iron catalysis has been successfully implemented for reductive cross-coupling of activated esters (Fig. 1c). However, such transformations remain scarce, underscoring the importance of developing new catalytic systems to overcome current limitations.
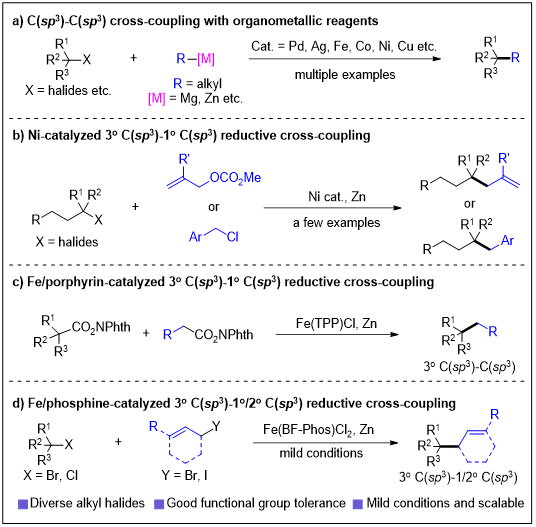
Fig. 1: Transition-metal catalyzed C–C cross-coupling for the construction of quaternary carbon centers
The research group of Prof. Shou-Fei Zhu at Nankai University has made continuous efforts toward iron-catalyzed organic transformations, designing a series of iron complexes supported by phenanthroline-nitrogen and cyclopropane-based phosphine ligands. Their work has enabled diverse transformations including hydrofunctionalization, carbozincation, and C–C coupling of unsaturated hydrocarbons. These studies revealed a close correlation between iron catalyst activity and spin state, leading to the proposition of "spin-responsive catalysis" as a novel dimension for reaction control. Building upon these foundations, the group has now reported an iron/ phosphine-catalyzed reductive coupling of tertiary alkyl halides with allyl halides, successfully forming 3o C(sp3)–1/2o C(sp3) bonds to construct various all-carbon quaternary centers (Fig. 1d). Notably, the 3o C(sp3) with 2o C(sp3) reductive coupling is previously unaccessible. Mechanistic studies indicate that carbonyl coordination assists the iron catalyst in overcoming substantial steric constraints and suppressing competitive pathways, serving as the key to reaction success. This work further demonstrates the significant potential of iron catalysts in facilitating highly challenging coupling reactions through right choice of the ligands. Relevant achievements were published in Nat. Commun. 2025, DOI: 10.1038/s41467-025-64781-5.