C-Glycosides have garnered significant attention in pharmaceutical research due to their superior metabolic stability compared to O- and N-glycosides. However, the direct and efficient synthesis of unprotected C-glycosides remains challenging, particularly for thermodynamically less stable furanose-type C-glycosides. Recently, Professor Gang He’s team at Nankai University developed a biomimetic "open-and-close" strategy to directly synthesize 1,2-cis-alkenyl furanosyl C-glycosides from native unprotected aldoses. This method features several highlights: (1) direct utilization of unprotected aldoses, eliminating protection/deprotection steps; (2) broad substrate scope compatible with various unprotected aldoses and alkenylboronic acids; (3) operational simplicity, mild conditions, high stereoselectivity (α/β up to >20:1), and scalability to gram-scale; (4) versatile downstream transformations of alkenyl glycosides enabling rapid access to challenging 1,2-cis-alkyl furanosyl C-glycosides. This work provides a new approach for synthesizing unprotected furanosyl C-glycosides and advancing C-glycoside-based drugs and functional materials.
The precise synthesis orchestrated by Nature's "molecular machines" has provided chemists with crucial inspiration for developing novel synthetic strategies. Inspired by enzymatic C-nucleoside biosynthesis (Fig. 1a), Professor Gang He's team developed a biomimetic "open-and-close" strategy: Specifically, morpholine mimics the role of lysine residues in C-glycoside synthase to form linear iminium intermediates with aldoses, which then undergo Petasis reactions with alkenylboronic acids to generate 1,2-anti-linear polyhydroxyamines with high selectivity. Subsequently, the highly electrophilic difluorocarbene selectively activates the C–N bond, promoting intramolecular SN2 substitution where the C4 hydroxy group attacks the anomeric carbon, efficiently constructing 1,2-cis-furanosyl C-glycosides through an "open-ring/close-ring" process (Fig. 1b).
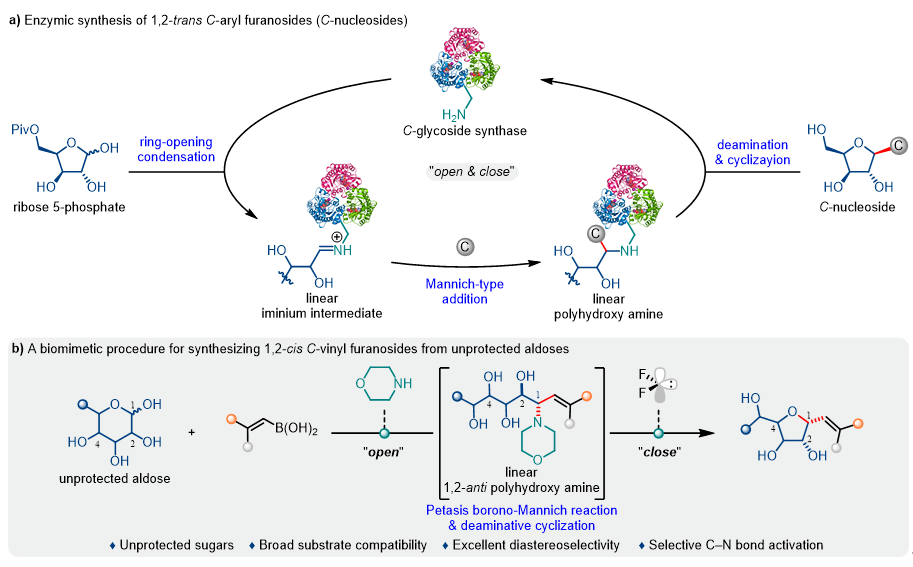
Figure 1. Background Introduction and Reaction Design
This method demonstrates excellent substrate generality. A wide range of aryl/heteroaryl vinylboronic acids, linear/cyclic/multi-alkyl substituted alkenylboronic acids, as well as alkynyl boron reagents and arylboronic acids are all compatible, affording target products in good yields with good-to-excellent diastereoselectivity (Fig. 2a). Furthermore, the protocol proves applicable for glycosylation of natural products like estrone and pharmaceutical molecules (Fig. 2b), while maintaining broad compatibility with various native mono- and disaccharides (Fig. 2c).
The strategy also exhibits promising synthetic utility (Fig. 2d), enabling one-pot reactions, gram-scale preparations, and downstream derivatizations of furanosyl C-glycosides. Notably, employing the team's biomimetic "open-and-close" approach, thermotropic liquid crystal materials can be prepared at gram scale from commercially available L-xylose in just 3 steps (Fig. 2e). This represents a significant improvement over traditional routes requiring 9 steps starting from protected D-glucose under cryogenic conditions with lithium reagents, providing a more efficient access to such architectures.
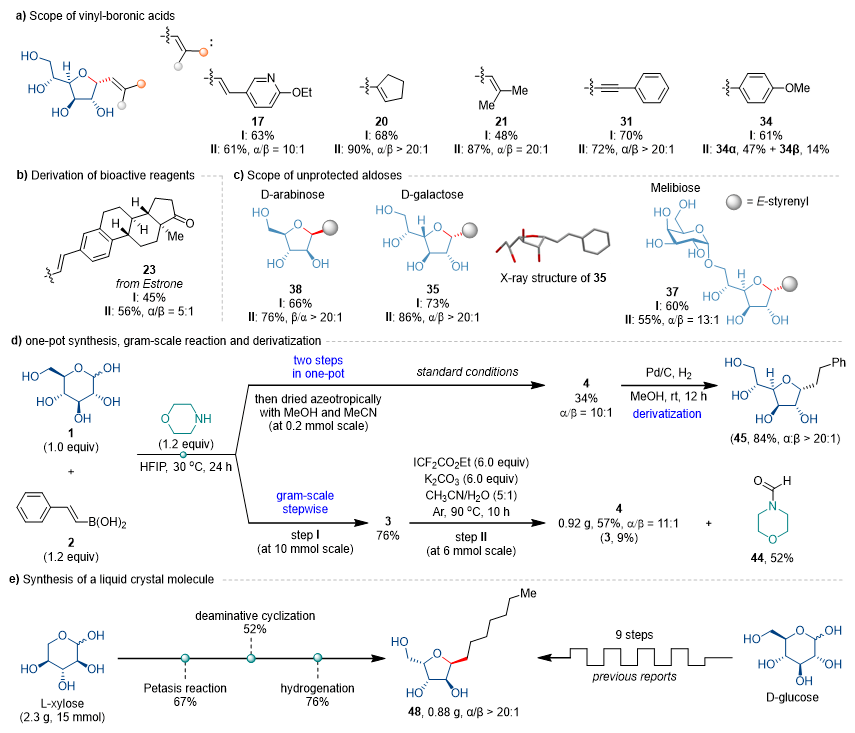
Figure 2. Substrate Scope and Synthetic Applications
Control experiments (Fig. 3a) provided crucial mechanistic evidence: 1) Deuterium labeling studies using D2O showed no deuteration in the main product, with the deuterated byproduct (44-D) originating from intermediate hydrolysis; 2) Difluorocarbene trapping experiments dramatically suppressed cyclization, while isolation of product 49 confirmed difluorocarbene involvement. Based on these results and prior reports, the authors proposed a plausible mechanism featuring three key stages: 1) Ring-opening and C–C bond formation: Morpholine forms an iminium intermediate with aldose, enabling vinyl migration via TS-I transition state; 2) C–N bond activation: Sequential difluorocarbene reaction with the morpholine moiety generates ylide Int-II and quaternary ammonium salt Int-III, activating the allylic C–N bond; 3) SN2 ring-closure: Intramolecular SN2 cyclization by the C4 hydroxyl yields 1,2-cis-furanosyl C-glycosides with inverted anomeric configuration (Fig. 3b).
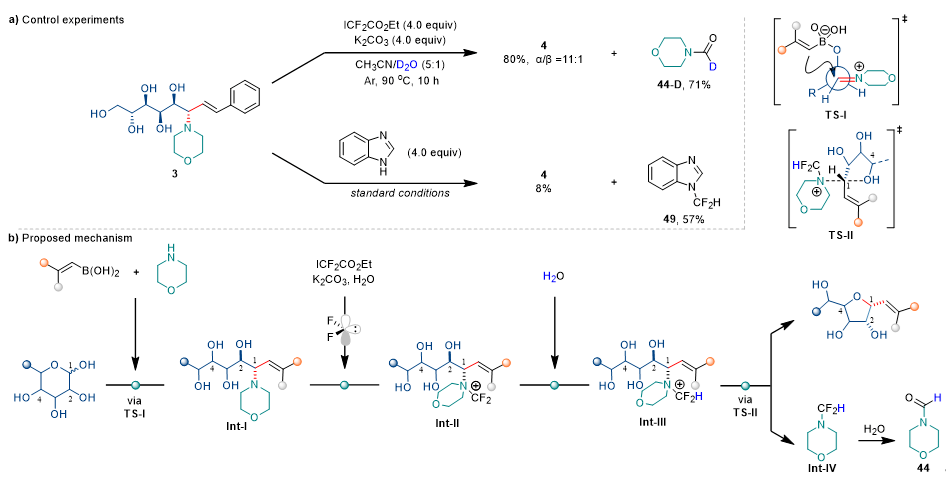
Figure 3. Mechanistic Study
In summary, Professor Gang He's team at Nankai University developed a biomimetic "open-and-close" strategy for efficient synthesis of 1,2-cis-alkenyl furanosyl C-glycosides directly from unprotected aldoses. The strategy's core involves: 1) "Opening": Morpholine mimics lysine residues in C-glycoside synthase to mediate aldose ring-opening and Petasis reaction with alkenylboronic acids for C–C bond formation; 2) "Closing": Difluorocarbene activates the C–N bond to trigger intramolecular SN2 deaminative cyclization, ultimately delivering 1,2-cis-alkenyl furanosyl C-glycosides efficiently.
This work was recently published in the Journal of the American Chemical Society, with Professor Gang He as corresponding author. Dongyang Sun (2022 PhD candidate) served as first author, and Xuerui Dou (Master's student) made significant contributions. Relevant achievements were published in J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c14877.
Professor Gang He
Professor and doctoral supervisor at Nankai University. He received his bachelor's degree from the College of Chemistry at Nankai University and his Ph.D. from the Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences (SIOC). From 2010 to 2014, he conducted postdoctoral research at The Pennsylvania State University, USA. In 2015, he joined the State Key Laboratory of Elemento-Organic Chemistry at Nankai University and was promoted to full professor in 2021. His research focuses on organic chemistry, particularly methodologies for the green and efficient construction of C-glycosidic scaffolds. He has published multiple SCI-indexed papers in leading journals such as Nature Chemistry, Nature Catalysis, J. Am. Chem. Soc., and Angewandte Chemie International Edition, and holds three authorized Chinese patents.
A Biomimetic Procedure for the Synthesis of 1,2-cis C-Vinyl Furanosides from Unprotected Aldoses
Dongyang Sun, Xuerui Dou, Fen Wang, Jiawei Wu, Wenbo Wang and Gang He*
J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c14877