Professor Qingmin Wang's group at Nankai University developed a photocatalytic method using readily available silylboronic pinacol esters to synthesize antifungal gem-difluoroallylsilane compounds through a radical transfer strategy, overcoming their high oxidation potential limitation. Biological assays confirmed that several target compounds exhibited moderate to potent activity against plant pathogens including Botrytis cinerea, Setosphaeria turcica, and Rhizoctonia solani. Relevant achievements were published in Chinese Chemical Letters 2025, DOI: 10.1016/j.cclet.2025.111940.
Research Breakthrough
Plant pathogenic fungi pose severe threats to global food security, ecosystems, and human livelihoods. As green chemical control remains the most mainstream and efficient management strategy, the scarcity of novel targets and lead compounds has become a critical bottleneck in fungicide development. Consequently, the efficient discovery of new fungicidal scaffolds and novel targets is of paramount importance. Fluorine—the element with the second smallest atomic radius and highest electronegativity—forms exceptionally strong bonds with carbon. Incorporating fluorine into bioactive molecules can profoundly alter their acidity, lipophilicity, and stability, thereby enhancing biological activity through improved target binding affinity, optimized molecular transport efficiency, and resistance to metabolic degradation.
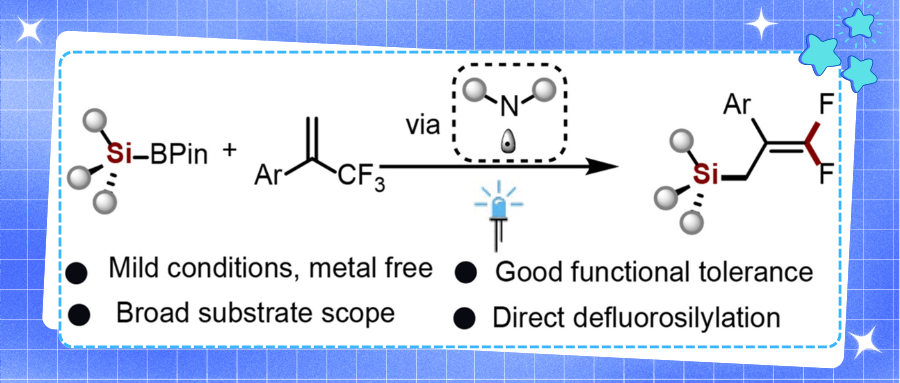
While silicon derivatives serve as crucial precursors in related fields, the high oxidation potential of silylboronic pinacol esters has limited their photochemical silylation applications. Conventional approaches rely on nucleophilic reagents to modulate redox potentials, whereas amine radicals emerge as an innovative strategy demonstrating Si-B bond activation potential for sustainable synthesis. Currently, silicon and gem-difluoroalkene moieties predominantly exist separately in most compounds. However, integrating both distinctive structural motifs into single molecules may provide medicinal chemists with transformative perspectives given their unique configurations and pharmaceutical applications.
Figure 1. Reaction Design
Addressing the critical challenge of fungicide lead scarcity, the authors employed photocatalytic radical approaches to efficiently construct bioactive gem-difluoroalkene derivatives (Fig. 1).This synthetic protocol features mild and environmentally benign conditions with broad substrate scope, accommodating diverse functional groups and natural product architectures while enabling gram-scale production. Selected compounds demonstrated superior antifungal activity against Botrytis cinerea (Table 1), with DFT and molecular electrostatic potential (MEP) analyses elucidating key structure-activity relationships (Fig. 2).
Table 1. Bioassay Data
Figure 2. DFT and MEP Analysis
Summary
The authors developed an efficient method to generate silyl radicals through homolytic substitution reactions of silylboronic pinacol esters with amine radicals, simultaneously incorporating gem-difluoroalkene fragments. This approach enables the scalable synthesis of silicon-containing difluoroalkene compounds under mild conditions, demonstrating remarkable antifungal activity (>60% inhibition rate at 50 μg/mL against pathogens like Botrytis cinerea), with EC₅₀ values surpassing or matching commercial fungicides. DFT and molecular electrostatic potential (MEP) analyses revealed unique electronic characteristics of high-activity compounds, while scanning electron microscopy confirmed their ability to effectively inhibit fungal mycelial growth. This study provides innovative lead compounds for developing structurally optimized fungicides with novel mechanisms.
Corresponding author Qingmin Wang (Professor, Nankai University) led the research, with Yue Fuyang, Yuan Fei, and Li Kun (Ph.D. candidates) as co-first authors. Support was provided by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and the Frontiers Science Center for New Organic Matter at Nankai University.
Research Profile of Prof. Qingmin Wang's Group

The research group led by Professor Qingmin Wang operates within the National Key Laboratory of Elemento-Organic Chemistry, the Frontiers Science Center for New Organic Matter, and the College of Chemistry at Nankai University. Currently comprising over 20 faculty members and graduate students, the team focuses on eco-friendly agrochemical/drug discovery, functional additives development, and sustainable synthetic methodologies. The group has undertaken 40+ research projects funded by national and municipal agencies, including the Ministry of Science and Technology, National Natural Science Foundation of China, and Tianjin Municipal Government. With 300+ SCI publications in journals such as Science Advances, Nature Communications, Angewandte Chemie, and Journal of Medicinal Chemistry, the team holds 100+ authorized patents across China, the U.S., and Europe. Their innovations include biomimetic pyrethroid pesticides, novel antiviral agrochemicals, and green acaricides—several undergoing industrial development—along with Category I new drug candidates. Seven academic books/chapters have been published, while developed clean production technologies for fine chemicals have been commercially implemented with significant economic impact. The group has trained 100+ visiting scholars, postdocs, and graduate students (Ph.D./M.S.).