The "Magic Methyl Effect" holds tremendous potential in medicinal chemistry, where methylating key drug scaffolds serves as a powerful tool for optimizing pharmaceutical properties. Pyridine, being one of the most prevalent heterocycles in drug molecules, has long been a focal point in synthetic chemistry for its functionalization. However, due to the strong electron-withdrawing effect of the pyridine nitrogen atom, significant progress has been made in introducing methyl groups at the ortho- or para-positions. In stark contrast, achieving highly selective methylation at the meta-position remains a formidable challenge.
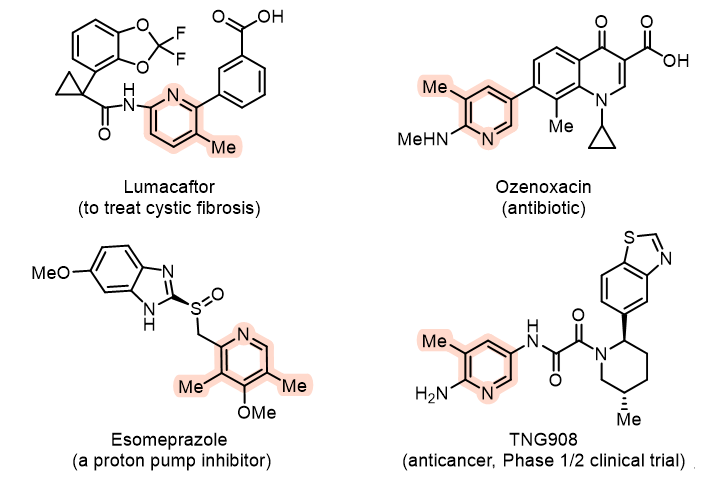
Figure 1. Drugs containing methyl-substituted pyridines
In recent years, the research group of Wang Xiaochen at Nankai University has developed a novel strategy for meta-functionalization of pyridines: First, organoboron-catalyzed hydroboration of pyridine generates a dihydropyridine intermediate with highly nucleophilic β-site reactivity; subsequently, this intermediate undergoes substitution reactions with electrophiles to form functionalized dihydropyridines; finally, under the action of oxidants, the dihydropyridine undergoes aromatization to yield meta-functionalized pyridines. Based on this strategy, the research group successfully achieved reactions between pyridines and imines or electron-deficient aldehydes/ketones at the meta-position (J. Am. Chem. Soc. 2022, 144, 4810), and subsequently extended the methodology to include meta-trifluoromethylthiolation, meta-difluoromethylthiolation, meta-cyanation, and metal-controlled asymmetric allylation (J. Am. Chem. Soc. 2022, 144, 14463; Angew. Chem. Int. Ed. 2023, 62, e202216894; J. Am. Chem. Soc. 2023, 145, 11789; Angew. Chem. Int. Ed. 2023, 62, e202307697). Recently, the team successfully applied this strategy to achieve meta-methylation and meta-alkylation of pyridines, with related findings published in J. Am. Chem. Soc.
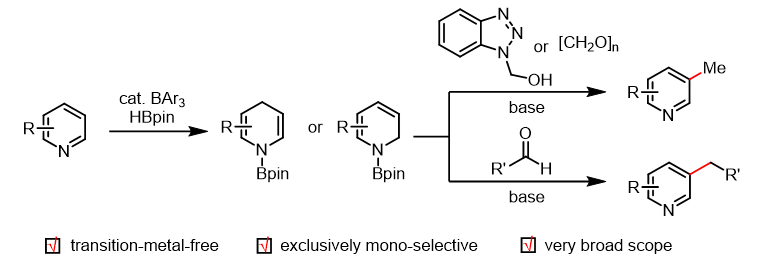
Figure 2. meta-C-H methylation and alkylation of pyridines via dihydropyridines (New methodology)
The research team employed (1H-benzo[d][1,2,3]triazol-1-yl)methanol as a formaldehyde surrogate (with formaldehyde serving as the electrophile) to efficiently achieve meta-methylation of pyridines through a sequence of substitution, deoxygenative elimination, and aromatization steps. This methodology demonstrates broad substrate compatibility, accommodating pyridine derivatives bearing substituents at C2, C3, or C4 positions as well as polysubstituted pyridines. Notably, the authors successfully accomplished late-stage functionalization of various pyridine-containing pharmaceutical molecules, achieving precise meta-methyl modifications in all cases. Furthermore, the use of deuterated paraformaldehyde enabled convenient incorporation of deuterated methyl groups.
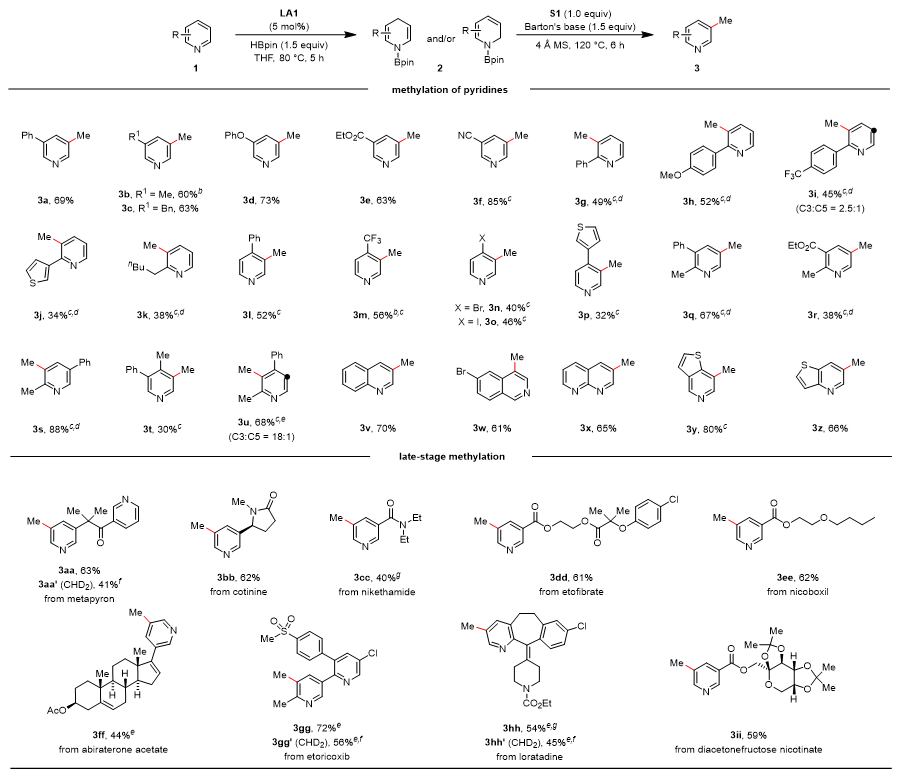
Figure 3. Scope of meta-Methylation with Respect to the Pyridine Substrate
Furthermore, this strategy has been successfully extended to aldehyde compounds as electrophiles, enabling the meta-alkylation of pyridines. Both aromatic aldehydes and aliphatic aldehydes, including chiral natural product-derived aldehydes, participate smoothly in the reaction, demonstrating broad substrate scope and excellent functional group compatibility.
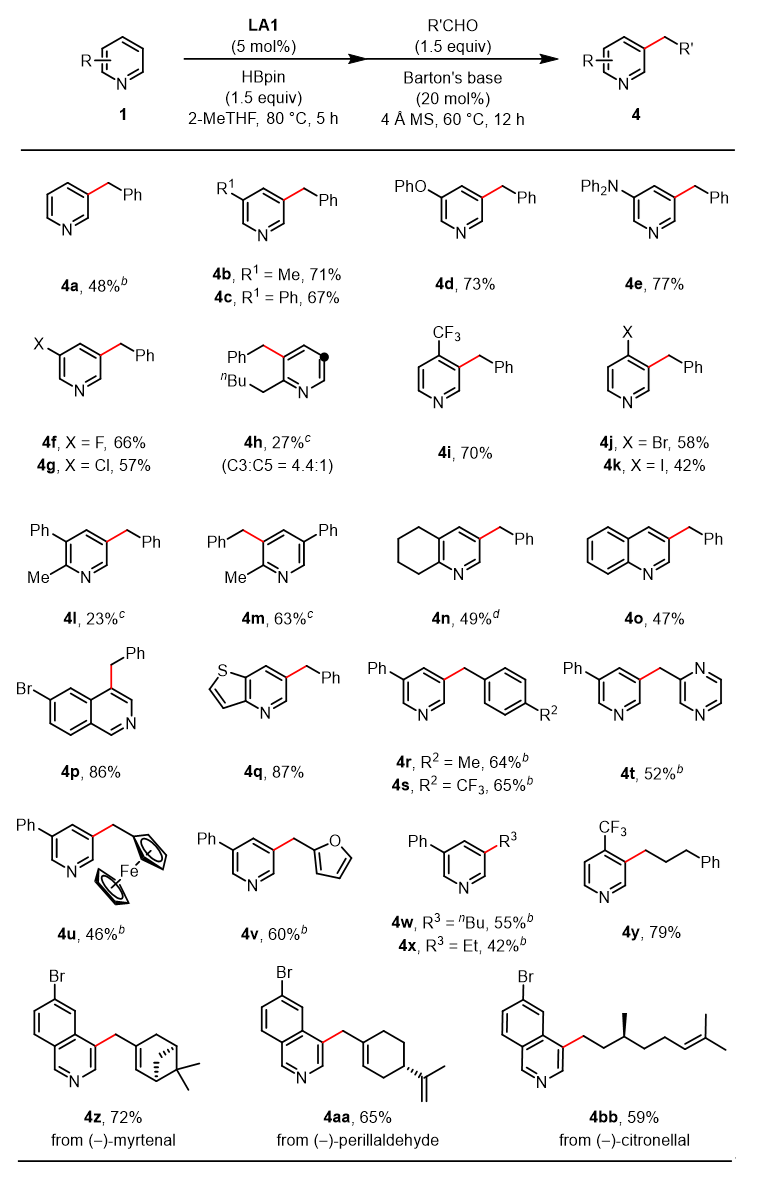
Figure 4. meta-Alkylation Reactions of pyridines with Aldehydes
Additionally, through combined density functional theory (DFT) calculations and mechanistic investigations, the authors elucidated the detailed reaction mechanism. The process involves several key steps: nucleophilic addition, proton transfer, deoxygenative elimination, and aromatization. Among these, experimental kinetic studies confirmed that the deoxygenative elimination step serves as the rate-determining step of the reaction.
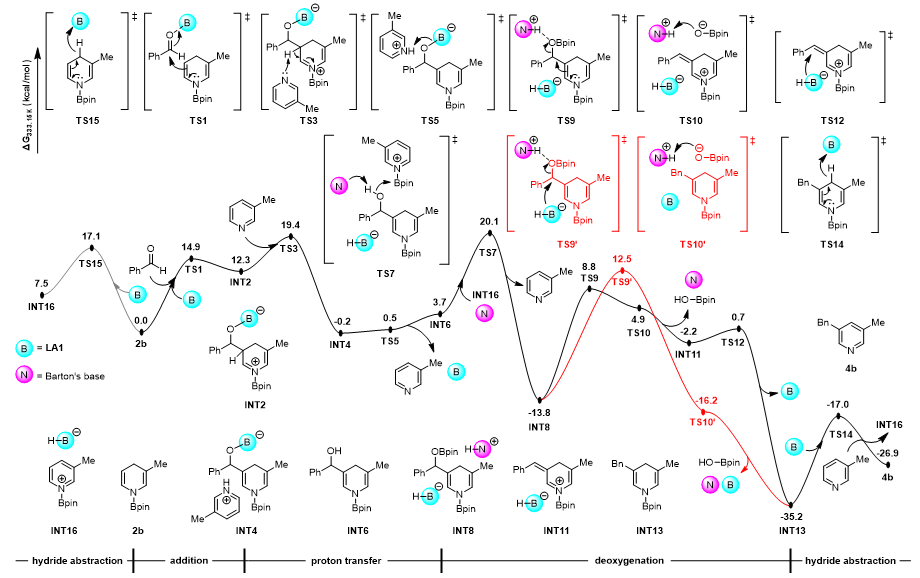
Figure 5. Gibbs free energy profile for the reaction between dihydropyridine 2b and benzaldehyde, computed at the SMD(THF)/M06-2X-D3(0)/ def2-TZVPP//PCM(THF)/ωB97X-D/6-31G(d) level of theory
In summary, the research group led by Professor Xiaochen Wang has developed an organoboron-catalyzed method for the meta-methylation and alkylation of pyridines, providing a novel strategy for pharmaceutical synthesis and drug development.
PhD candidates Zhihao Chen and Lu Liu are the co-first authors of this paper. This work was financially supported by the National Key Research and Development Program of China and the National Natural Science Foundation of China.
For more information:
Methylation and Alkylation of Pyridines at the meta Position Using Aldehydes or Aldehyde Surrogates
Zhi-Hao Chen, Lu Liu, Yun-Bo Wang, Heng Luo, Zi-Lu Tang, Xin-Yue Zhou, Xiao-Chen Wang*
J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c13428