Azaarene moieties substituted with alkyl groups containing carbon stereogenic centers are common in pharmaceuticals, agrochemicals, and natural products. Direct functionalization of azaarenes at the α-position of a linear alkyl substituent via deprotonation with a base and electrophilic trapping of the resulting carbanion is the most straightforward strategy for constructing such structures. However, the inherent weak acidity of α-protons restricts current methodologies primarily to heteroarenes containing strong electron-withdrawing groups (EWGs). For non-activated heteroarenes lacking EWGs, deprotonation necessitates strong bases, which often promote competitive background reactions and may lead to decomposition of chiral catalysts, posing significant challenges for asymmetric catalytic transformations.
Hydrometalation of conjugated enynes generates π-allyl metal electrophiles in situ, enabling enantioselective construction of chiral allene frameworks via transition metal catalysis. Nevertheless, this strategy remains limited to highly acidic pronucleophiles. For weakly acidic non-activated alkyl heteroarenes, their deprotonation requires strong bases that generate proton-locked conjugate acids with insufficient acidity for proton transfer, thereby impeding the formation of reactive electrophilic species and rendering such substrates incompatible with existing catalytic systems.
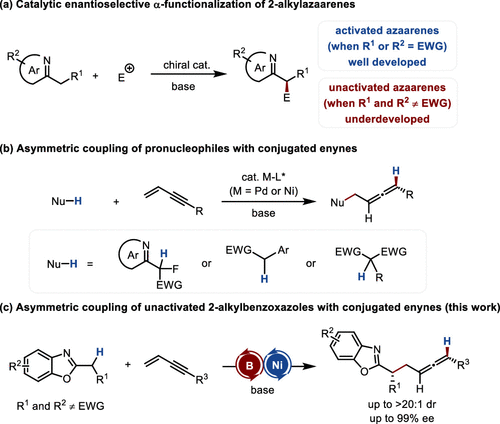
Fig. 1 Catalytic Enantioselective α-Functionalization of 2-Alkylazaarenes
Recently, Xiao-Chen Wang’s group demonstrated that boron-based catalysts can effectively activate the α-C–H bond of heteroarenes, increasing proton acidity (J. Am. Chem. Soc. 2024, 146, 24663–24669). By steric modulation, a dynamic coordination/dissociation equilibrium with substrates was achieved, ensuring catalytic turnover. Building on this, the Wang group now reports a boron/nickel cocatalytic system that enables the first asymmetric coupling of non-activated 2-alkylbenzoxazoles with conjugated enynes (Fig. 1). Relevant achievements were published in J. Am. Chem. Soc., 2025, DOI: 10.1021/jacs.5c11167.