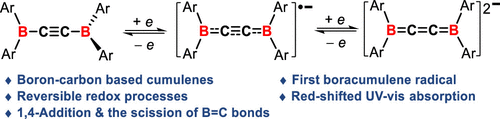
Cumulenes feature a linear chain of sp-hybridized carbon atoms terminated by two sp2-hybridized carbon centers. They are classified as odd- or even-numbered based on cumulative double-bond parity, with only odd-numbered cumulenes exhibiting coplanarity between terminal groups. Owing to their unique consecutive double bonds, synthetic versatility for condensed π-conjugated systems, and potential applications in the material science, cumulenes have been widely investigated in recent years. Replacement of one or more carbon atoms in the cumulenes with boron atoms furnishes the boron-containing cumulene systems. This strategy could efficiently modulate their electronic natures and thus expand their applications, ranging from synthetic chemistry to material science. In boron chemistry, the boron π-species, such as borenes and borataallenes, have been studied over the past years, while the relatively long π-conjugated chains (cumulenes) are still less explored.
Recently, Chunming Cui’s group have synthesized the first radical anionic and dianionic 1,4-diborabutatrienes 1•–K+(thf)2 and 12–(K+)2 by the sequential reduction of diborylacetylene 1 with KC8, and those processes proved to be reversible. Joint experimental and computational studies demonstrate their pronounced cumulene feature. Dianionic 12–(K+)2 exhibits remarkably red-shifted UV–vis absorption compared to the all-carbon tetraarylbutarienes. It undergoes 1,4-addition with phenylacetylene to generate 2, while the reaction with DipN3 leads to the formation of anionic iminoborane 3 via the complete scission of the B=C bonds in 12–(K+)2. Regarding the unique electronic structure and reactivity of B-embedded butatriene 12–(K+)2, these findings hold significant implications for the development of unconventional cumulenes possessing novel physical and chemical properties. Moreover, their results highlight that the reduction of boryl alkyne is an innovative protocol for the construction of boron-containing cumulenes, and the synthesis of longer boracumulene systems ([R2B=(C=C)n=BR2]2–, n = 2, 4, ...) is currently undertaken in their laboratory. Relevant achievements were published in J. Am. Chem. Soc. 2025, DOI: 10.1021/jacs.5c09195.