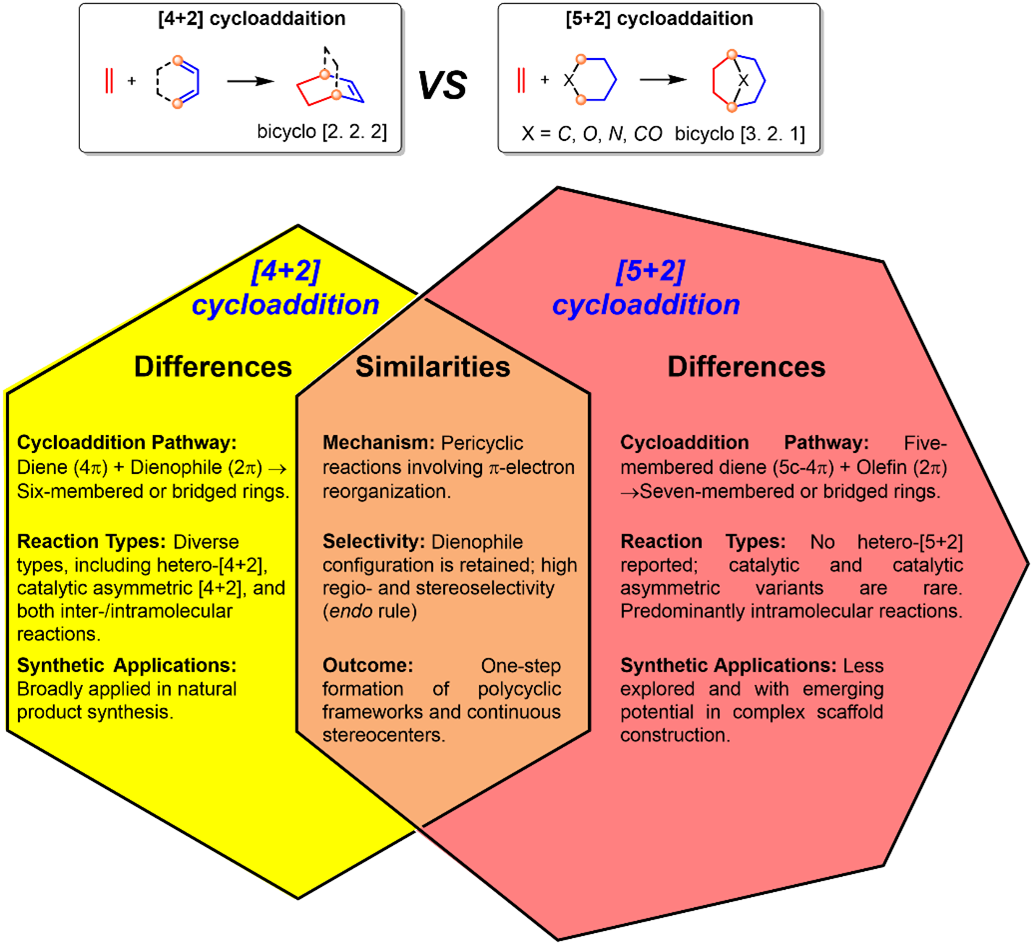
Cycloaddition reactions represent a powerful toolbox in organic synthesis for constructing complex ring systems. Among them, the [4+2] cycloaddition, exemplified by the Nobel Prize-winning Diels–Alder reaction, has been extensively applied in the synthesis of pharmaceuticals and functional materials. In contrast, [5+2] cycloadditions have emerged as a novel strategy enabling the one-step formation of seven-membered rings and highly strained bridged scaffolds, offering unique disconnections for complex natural product synthesis. However, unlike the well-established [4+2] paradigm, [5+2] cycloadditions remain in their infancy with limited exploration and practical applications thus far.
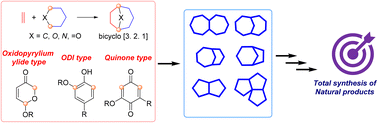
Recently, Jun Deng’s group have published a review article that begins by comparing the differences between [4+2] and [5+2] cycloadditions in terms of reaction mechanisms, substrate scope, stereoselectivity, and synthetic applications, with a focused discussion on three pivotal [5+2] reaction types: 1) oxidopyrylium ylide-based [5+2] cycloaddition, 2) oxidative dearomatization-induced (ODI) [5+2] cycloaddition, and 3) benzoquinone [5+2] cycloaddition. Through representative total synthesis case studies of natural products (e.g., (−)-colchicine, (−)-retigeranic acid A, and merocytochalasan), the article elaborates on the design principles, reaction mechanisms, and unique advantages of each reaction type in constructing complex molecular frameworks. By directly comparing [5+2] cycloadditions with [4+2] cycloadditions and other synthetic strategies, the authors highlight both the strengths (e.g., step economy, stereocontrol capability, and bridged-ring formation) and limitations of [5+2] reactions. In contrast to the well-established [4+2] cycloadditions, [5+2] cycloadditions still present vast unexplored potential, particularly in asymmetric catalysis, substrate generality, and late-stage applications in natural product synthesis. Going forward, [5+2] cycloadditions are poised to emerge as a transformative tool for advancing total synthesis of natural products, drug discovery, and functional materials innovation. Relevant achievements were published in Nat. Prod. Rep., 2025. DOI: 10.1039/D5NP00023H.