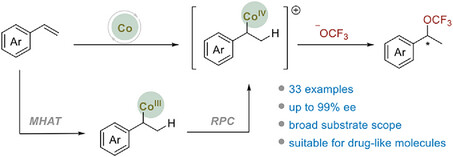
The trifluoromethoxy group has garnered increasing attention in pharmaceutical and agrochemical chemistry due to its ability to enhance the metabolic stability and lipophilicity of drugs. It is well established that drugs require optically pure enantiomers, making the development of methods for constructing chiral trifluoromethoxy compounds highly desirable. However, despite the numerous racemic conversions reported to date, research into enantioselective versions remains in its early stages, with only a few studies published. Notably, benzylic functional groups are prevalent in bioactive molecules, developing strategies for synthesizing chiral benzylic trifluoromethoxy compounds are thus significant as they will contribute to the progress of drug development. To date, several systems have been reported to synthesize racemic benzylic trifluoromethoxy molecules. However, these reactions either lack a chiral source or fail to control enantioselectivity. Only two enantioselective versions have been successfully developed. The key to constructing chiral benzylic trifluoromethoxy compounds via direct asymmetric trifluoromethoxylation lies in finding a system that: (1) exhibits high reactivity, due to the facile decomposition of OCF3; (2) can generate strong electrophilic intermediates, given the weak nucleophilicity of OCF3; and (3) can be modulated by steric ligands so as to induce enantioselectivity around the less sterically hindered OCF3 group. Through extensive research, we observed that Co-catalyzed asymmetric hydrogen functionalization of olefins with various nucleophiles have emerged as a promising strategy to construct benzyl compounds. In particular, in situ generated alkylcobalt(IV) intermediates through Co-H catalyzed hydrogen atom transfer (HAT) and subsequent radical polar crossover (RPC) process, have shown electrophilicity and the ability to undergo stereospecific SN2-displacement with nucleophiles, offering opportunities for asymmetric induction. Notably, the outer sphere mechanism may also help avoid competitive β-F elimination, which has been observed in inner-sphere OCF3-coordinated transition metal species.
Recently, Pingping Tang’s group have successfully developed the first Co-catalyzed enantioselective intermolecular hydrotrifluoromethoxylation reaction between aromatic alkenes and TFMS. This method offers mild reaction conditions, broad functional group tolerance, and an extensive substrate scope. Under optimized conditions, they achieved the synthesis of various chiral benzylic trifluoromethoxy compounds with yields reaching up to 77% and enantiomeric excesses of up to 99%. Additionally, the hydrotrifluoromethoxylation of alkenes derived from valuable bioactive molecules was also accomplished, highlighting the potential application of this method in medicinal chemistry. Relevant achievements were published in Angew. Chem. Int. Ed., 2025. DOI: 10.1002/anie.202501680.