Selective modulation of catalytic reactions represents a core research focus on organic synthesis. The control of reaction selectivity (including chemo-, regio-, and stereoselectivity) is primarily governed by the catalyst and the stereoelectronic properties of the substrate. However, numerous studies have demonstrated that additives can also significantly influence reaction selectivity. In certain instances, different additives can lead to products with markedly distinct selectivities. This observation suggests that additives offer a promising and cost-effective strategy for modulating reaction selectivity. A comprehensive understanding of the mechanisms underlying additive effects is crucial for the rational design of reactions. Despite this potential, systematic studies on the effects of additives are scarce, and the mechanistic insights into their action remain limited.
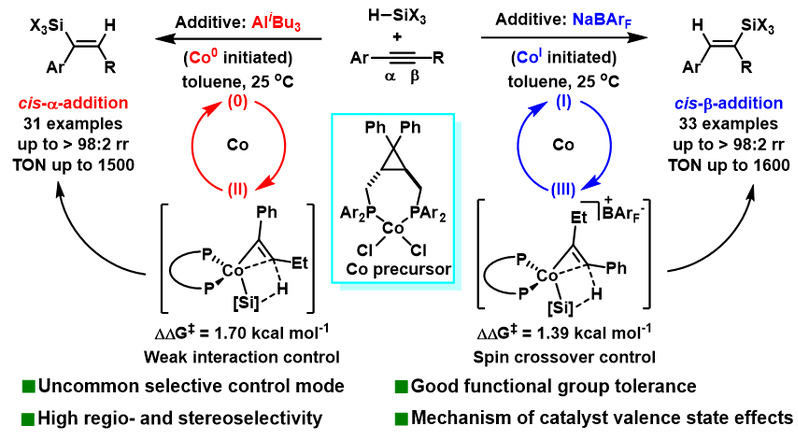
Recently, Shou-Fei Zhu’s group have reported a Co-catalyzed hydrosilylation of internal alkynes in which the regioselectivity was predominantly determined by the additives. The reaction using AliBu3 as an additive exhibited cis-α-selectivity, while that of NaBArF exhibited cis-β-selectivity. Additionally, the highly cis-β-selective hydrosilylation of dialkyl and silyl-alkyl internal alkynes was realized using AlEt3 as an additive, effectively distinguishing subtle differences in substituents at both ends of the alkynes (e.g., ethyl vs methyl). These Co-catalyzed hydrosilylation reactions featured mild conditions, simple operation, broad substrate applicability, good functional group compatibility, and high efficiency, offering significant potential for application. The mechanistic experiments showed that different additives generated active Co catalysts with varying valence states, leading to the catalyst experiencing different spin states and ultimately resulting in regio-divergence. This research not only provides an efficient method for synthesizing trisubstituted alkenyl silicon compounds but also uncovers novel additive effects and new approaches for regulating regioselectivity by altering the valence state of metal catalysts. These findings offer new insights into the design of catalysts and catalytic reactions. Relevant achievements were published in CCS Chemistry, 2025. DOI: 10.31635/ccschem.025.202405338.