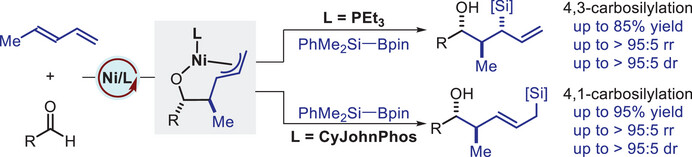
1,3-Dienes are highly versatile in synthetic chemistry, capable of undergoing numerous transformations. Difunctionalization of 1,3-dienes is a powerful strategy for introducing multiple functional groups in a single step, greatly simplifying the synthesis of synthetically useful allylic compounds. Typically, these reactions are facilitated by transition metal catalysts through π-allyl-metal intermediates, which can interact with both electrophiles and nucleophiles. However, the regioselectivity of these reactions is influenced by the stability and reactivity of the intermediates and is often complicated by allylic rearrangements. Despite advances in catalytic systems for regioselective difunctionalization, developing catalyst-controlled regiodivergent approaches that overcome substrate limitations-yielding diverse, pure regioisomeric, and stereoisomeric products—remains challenging. Carbosilylation of 1,3-dienes is a compelling method for synthesizing allylsilanes, which are valued in organic chemistry for their low toxicity and high stability. These compounds play a crucial role in various synthetic transformations and are used as monomers in the synthesis of silicon-containing polymers. Despite its significant potential, carbosilylation of 1,3-dienes has not been extensively explored. Achieving catalyst-controlled regiodivergent carbosilylation of 1,3-dienes remains a significant challenge. This difficulty stems from the complex control required over multiple potential reaction pathways, and, to date, no successful instances of catalyst-controlled regiodivergent carbosilylation of these compounds have been reported.
Recently, Li-Jun Xiao’s group have developed a novel ligand-controlled, regiodivergent nickel-catalyzed carbosilylation of 1,3-dienes with aldehydes and silylboranes, achieving unprecedented site selectivity. The use of PEt3 promotes 4,3-carbosilylation, whereas CyJohnPhos leads to 4,1-carbosilylation. This reaction exhibits high efficiency, exceptional regio- and diastereoselectivity, a broad substrate scope, and significant tolerance to various functional groups. The method provides a practical and efficient pathway for synthesizing valuable functionalized allylsilanes, which are essential for applications in complex natural product synthesis. These advancements open new possibilities for customizing complex reaction environments through ligand manipulation, setting the stage for further innovations in the selective catalytic transformation of 1,3-dienes. Relevant achievements were published in Angew. Chem. Int. Ed., 2025. DOI: 10.1002/anie.202504494.