Organometallic reagents have become indispensable tools in modern synthetic chemistry, with organolithium and organomagnesium reagents being among the most widely utilized main group species. Organoaluminum compounds based on the most abundant metal of Earth’s crust, however, present a compelling yet underexplored class of reagents with significant potential for further development. This limited utilization can be largely attributed to the paucity of efficient synthetic methodologies, which restrict the structural diversity and practical applicability of organoaluminum species. For example, although alkenylaluminum reagents are valuable intermediates for the synthesis of substituted alkenes, their direct preparation from alkenyl halides and aluminum is notoriously challenging. Alternatively, the transfer hydroalumination of alkynes offers a promising strategy to access these compounds but is often complicated by competing side reactions, including alumination, dihydroalumination, carboalumination, and cycloalumination. Furthermore, achieving high levels of regio- and stereocontrol in this transformation remains a significant challenge.
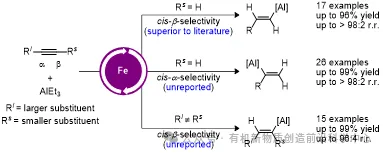
Recently, Shou-Fei Zhu’s group have developed a highly efficient iron-catalyzed transfer hydroalumination of alkynes characterized by high yields, excellent chemo-, stereo-, and regioselectivity, operational simplicity, mild reaction conditions, and broad functional group tolerance. This protocol allows for the high-efficiency preparation of various alkenylaluminum reagents, previously inaccessible by other methods, from commercially available AlEt3 and readily accessible alkynes. Iron catalysts bearing 2,9-aryl phenanthroline ligands enabled regiodivergent transfer hydroalumination of terminal alkynes, while those modified with 6,6′-diaryl-2,2′-bipyridine ligands facilitated cis-β-selective transfer hydroalumination of simple unsymmetrical internal alkynes. This work not only broadens the utility of organoaluminum reagents but also provides valuable insights into advancing iron catalysis. Relevant achievements were published in J. Am. Chem. Soc., 2025. DOI: 10.1021/jacs.5c02158.