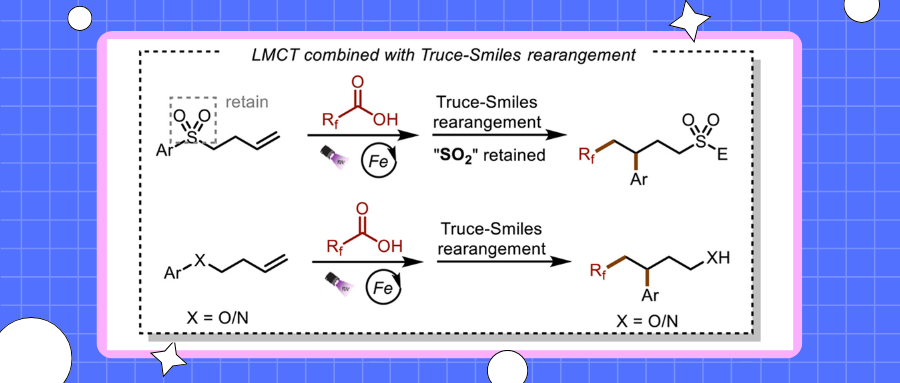
Of all the elements, fluorine has the highest electronegativity and the second smallest atomic radius. The incorporation of fluoroalkyl groups has emerged as a powerful tool for modulating the biological activity of pharmaceutical lead compounds. Compounds bearing these groups often show significantly better therapeutic efficacy than their parent molecules due to the enhanced metabolic stability, lipophilicity, and bioavailability of the former. In particular, the introduction of trifluoromethyl and difluoromethyl groups into drug and pesticide molecules has been increasing because these groups can serve as equivalents for methyl groups and other functional groups in later functionalization process. To introduce di- or trifluoromethyl groups into high-value compounds, chemists have developed various fluorination methods and reagents. Initially, reagents such as CF3I, CF3SO2Na, BrCF2H, and ClSO2CF2H were used, but the need for harsh reaction conditions and the instability of these reagents hindered their widespread application. To address these limitations, chemists developed a series of new fluorination reagents, including the Umemoto, Togni, and Hu reagents. These reagents are easier to use, but they show poor atom economy, sometimes require noble-metal-based photoredox catalysts, are highly oxidizing, and sometimes require sacrificial bases and/or high temperatures, features that limit their applicability. Radical rearrangements, such as the Truce-Smiles rearrangement, have received increasing attention because of their utility for the formation of chemical bonds via the migration of functional groups from one atom to another. Such rearrangements have been used for many important transformations. Since the Speckamp group reported the first free-radical-induced Truce-Smiles rearrangement in the 1970s, significant progress has been achieved, and Truce-Smiles rearrangements have been widely used for the bifunctionalization of alkenes and alkynes. In most of the published studies, sulfones and sulfonamides have been used as linkers for the bifunctional reagents. However, for thermodynamic reasons, after the migration reaction is complete, the sulfone group is released in the form of sulfur dioxide. As is well known, the release of sulfur dioxide into the atmosphere can lead to the formation of acid rain. Moreover, sulfur dioxide is carcinogenic, and emission in large quantities can have a deleterious impact on human health. Although human emissions are far inferior to volcanic emissions, reducing human emissions is still meaningful. Therefore, in terms of both atom economy and environmental friendliness, avoiding the generation of sulfur dioxide would be highly desirable.
Recently, Qingmin Wang’s group utilized earth-abundant, inexpensive iron as a catalyst and inexpensive, abundant, readily available polyfluoroalkyl carboxylic acids as substrates for sulfone-group-retaining Truce-Smiles rearrangement reactions that were mediated by ligand-to-iron charge transfer. The high oxidation potential of the fluoroalkyl carboxylic acids was overcome by using the iron catalyst; and sulfur dioxide release, which occurs in traditional Truce-Smiles rearrangement reactions, was avoided by adding water to the reaction mixture. Using this mild, operationally simple method, they synthesized a series of polyfluoroalkylated aromatic sulfones, amines, and alcohols from heteroaromatic hydrocarbons and electrophiles. Relevant achievements were published in Green Synth. Catal., 2025. DOI: 10.1016/j.gresc.2025.02.002.