Highly functionalized bicyclo[3.2.1]octane motifs are prevalent in pharmaceuticals and serve as core substructures in numerous natural products. Recent examples include asperones A (1) and B (2), two dimeric polyketides derived from the fungus Aspergillus sp. strain, which were isolated from centipede intestines by Yang and Kong (Scheme 1A). Structurally, both asperones A and B feature a highly oxidized bicyclo[3.2.1]octane core and are diastereomers. Biologically, asperones B, C, and D have demonstrated significant inhibition of nitric oxide (NO) production in lipopolysaccharide (LPS)-induced RAW 264.7 macrophage cells, suggesting their potential as lead anti-inflammatory agents. The biosynthesis of asperones A and B is proposed to involve quinone 9 and demethyl-gregatin A (12) through a double Michael addition (Scheme 1B). Although quinone 9 has not yet been isolated, we propose that it is derived from linear polyketide 6 via nonribosomal polyketide synthases (NR-PKSs) catalyzed Dieckmann condensation/reduction, decarboxylation, and subsequent oxidative decoration. Wang and Matsuda have shown that gregatin A (13) is derived from polyketide 10 through enzyme-catalyzed oxidative cyclization and methylation. An intriguing aspect of this biosynthetic pathway is the formation of furanone through a vinylogous internal nucleophilic substitution (SNi′), where the conjugated double bond migrates to an unconjugated position, forming a quaternary carbon center in an enantioselective manner. Stoloniferol B (7), the proposed biosynthetic precursor of quinone 9, was first synthesized by Hosokawa in 10 steps. Meanwhile, another key fragment gregatin A (13) was synthesized by Brückner in 7 steps from enantiomerically pure lactic esters. Based on biosynthetic network analysis, we hypothesized that asperones A (1) and B (2) could be assembled through a late-stage intermolecular [5+2] cycloaddition between demethyl-gregatin A (12) and p-quinone 9. To achieve the asymmetric synthesis of asperones A (1) and B (2), both demethyl-gregatin A (12) and p-quinone 9 must be prepared in enantiopure form to avoid the formation of potential diastereomers during the [5+2] cycloaddition. Taking these factors into account, we have envisioned a streamlined synthesis of asperones A (1) and B (2), starting with the enantioselective synthesis of both fragments, demethyl-gregatin A (12) and p-quinone 9.
Recently, Jun Deng’s group have accomplished the first asymmetric total synthesis of the anti-inflammatory polyketides asperones A (1) and B (2). Key synthetic steps include a Diels–Alder and retro-Diels–Alder cascade to construct the poly substituted phenol, an Al-Salen-catalyzed asymmetric cyanosilylation to form the tertiary alcohol of gregatin A, and an organocatalyzed intermolecular [5+2] cycloaddition of p-quinone with electron-deficient alkenes to build the crucial [3.2.1] octane core of asperones A (1) and B (2). Relevant achievements were published in J. Am. Chem. Soc., 2025. DOI: 10.1021/jacs.4c16252.
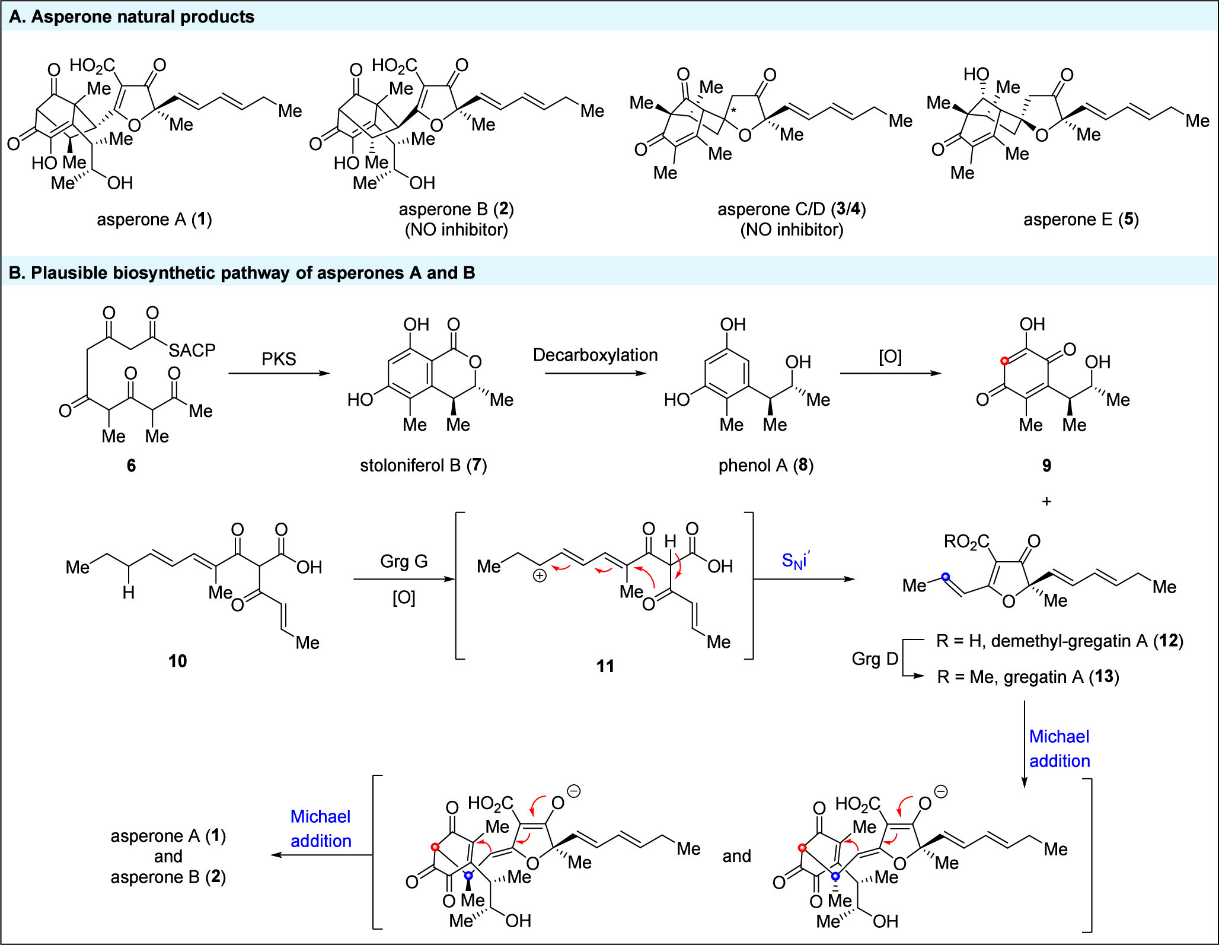
Scheme 1. Asperone Natural Products, Plausible Biosynthesis, and Previous Synthesis of Key Fragments
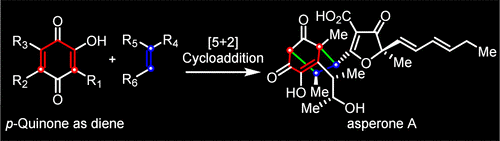
Graphic Abstract