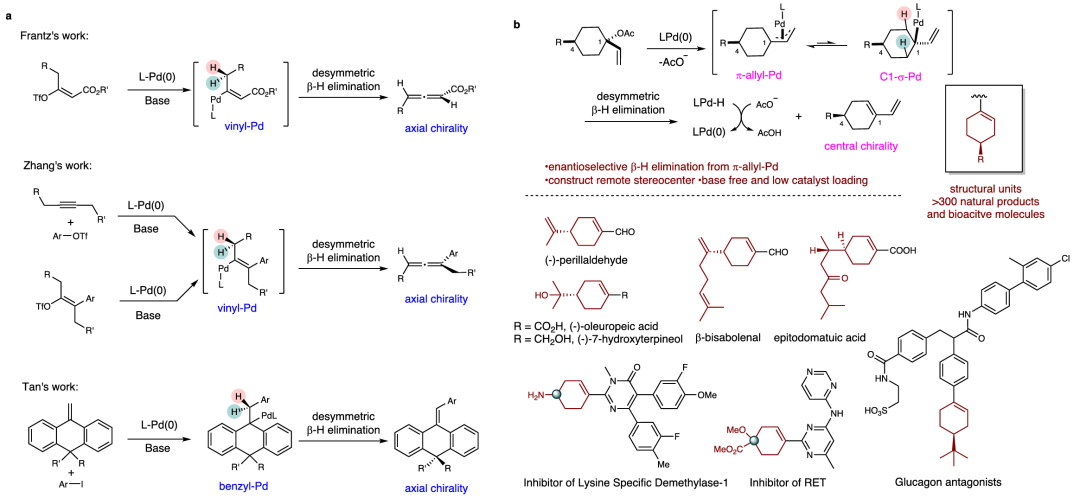
The β-hydride elimination is a fundamental chemical process that appears in many transition-metal catalyzed transformations, such as the Heck reaction, Saegusa oxidation, and migratory cross-coupling reactions. Chemists have carried out several experimental and theoretical studies to investigate the chemoselectivity and regioselectivity of β-hydride elimination. However, there have been very few attempts to study the stereoselectivity because this elimination step transforms two sp3 carbons into an alkene without generating a common stereocenter. The development of methods to achieve enantioselective β-hydride elimination is an attractive research topic in asymmetric transition-metal catalysis. Frantz and coworkers reported the first successful asymmetric β-hydride elimination from vinyl Pd(II)-complexes derived from enol triflates. This reaction features an asymmetric desymmetrization strategy, producing 1,3-disubstituted allenes with high enantioselectivities that bear axial chirality. Zhang et al. disclosed a Heck reaction-triggered β-hydride elimination, and their Pd-Xuphos system could furnish 1,1,3-trisubstituted chiral allenes. More recently, Tan et al. reported an elegant asymmetric Heck reaction of anthracenylidenes, which utilized the asymmetric β-hydride elimination to establish an axially chiral anthracenylidene skeletons28. Despite these achievements, the construction of central chiralities by asymmetric β-H elimination has never been addressed. The β-H elimination from the π-allyl-palladium species in the Tsuji-Trost reaction can lead to the formation of undesired product 1,3-dienes. However, this reaction has not been fully explored for its synthetic potential, particularly in enantioselective variants.
Recently, Weiwei Zi’s group have developed a palladium-catalyzed enantioselective desymmetric β-hydride elimination from π-allyl-Pd for the construction of remote stereocenters. This transformation features low catalyst loading, base-free conditions, and wide functional group tolerance. A series of 1,3-dienes bearing a C4-chiral center were synthesized efficiently from 1-vinylcyclohexyl acetates. Synthetic application of this method to the total synthesis of (-)-oleuropeic acid and (-)-7-hydroxyterpineol was demonstrated. DFT calculations indicated that the β-hydride elimination is both a rate-limiting and enantioselectivity-determining step. The non-covalent interactions between the chiral ligand and substrate play a key role in stereocontrol. Relevant achievements were published in Nat. Commun., 2025. DOI: 10.1038/s41467-025-57437-x.