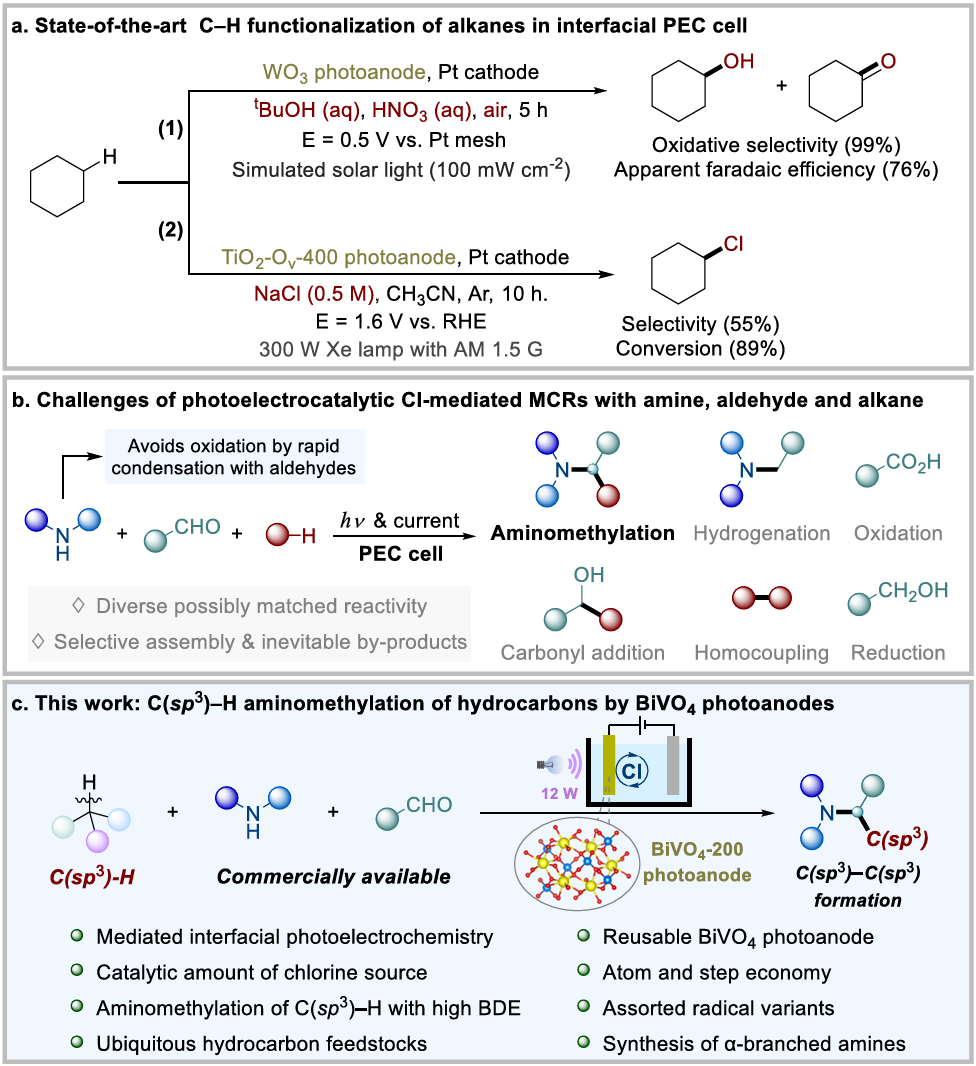
Interfacial photo electrochemistry (iPEC) at a semiconductor-liquid junction enables a resource-efficient and environmentally benign pathway for directly converting solar energy into chemical fuels, which has been widely explored for H2 production through water decomposition and reduction of carbon dioxide. However, their utility in organic synthesis has only been recognized in recent years and still faces constraints in terms of reaction diversity and underwhelming efficiency. In the last few decades, the swift advancement of photocatalysis and electrocatalysis has emerged as powerful tools, transforming the field of organic synthesis. In order to circumvent the energy barriers and achieve the organic conversion process in a more sustainable approach, photoelectrochemical catalysis has been introduced to many reported syntheses. Therein, iPEC, which uses immobilized photoelectrodes to replace dispersed catalysts in heterogeneous systems and soluble catalysts in homogeneous systems, avoids the requirement of a separation process and hence reduces cost. Additionally, certain homogeneous catalysts become inactive after the reaction concludes, rendering them unable to be recovered and reused. Moreover, the external potential of iPEC ensures fast charge separation and high reaction efficiency. Notably, the low and adjustable bias (even the absence of bias) in the iPEC cell saves energy input and avoids excessive oxidation, thereby minimizing the generation of byproducts. Despite these obvious advantages, iPEC for organic synthesis is still in its infancy, for example, the functionalization of C(sp3)–H of hydrocarbons with high bond dissociation energy (85-104 kcal mol–1) in iPEC has even fewer precedents. The Sayama group demonstrated that utilizing a WO3 photoanode under an oxygen atmosphere enables the oxidation of cyclohexane to cyclohexanol and cyclohexanone, attaining both outstanding selectivity for partial oxidation and excellent current utilization efficiency. After that, utilizing an oxygen-deficient TiO2 photoanode, the Duan group proposed a photoelectrochemical strategy to achieve efficient C–H halogenation of alkanes, producing various organic halides in high conversion. Although remarkable progress has been made, iPEC continues to encounter many challenges. For instance, (i) most proof-of-concept work has afforded unsatisfactory yields for the target products, (ii) there are limited applications for complex multi-component systems in organic synthesis, (iii) the photoanode materials often detach and deactivate during the reactions. Therefore, the development of a stable photoanode material and application of it in complex multi-component systems to construct high-value-added products is still largely unexplored and remains highly sought after.
Recently, Youai Qiu’s group have presented an efficient, stable, and recyclable BiVO4 photoelectrode material, with a focus on achieving Cl-mediated C(sp3)–H aminomethylation of alkanes with high bond dissociation energy (BDE) in a PEC cell. Their approach was carried out under mild conditions and does not require transition metals or oxidants. This transformation benefits from the use of commercially available reagents, high atom and step economy, and broad substrate scope. The proposed mechanism was grounded in the findings from the CV tests and mechanistic experiments. Due to its environmentally friendly and energy-efficient characteristics, they find the PEC technique particularly appealing for organic chemistry. Relevant achievements were published in Nat. Commun., 2025. DOI: 10.1038/s41467-025-57567-2.