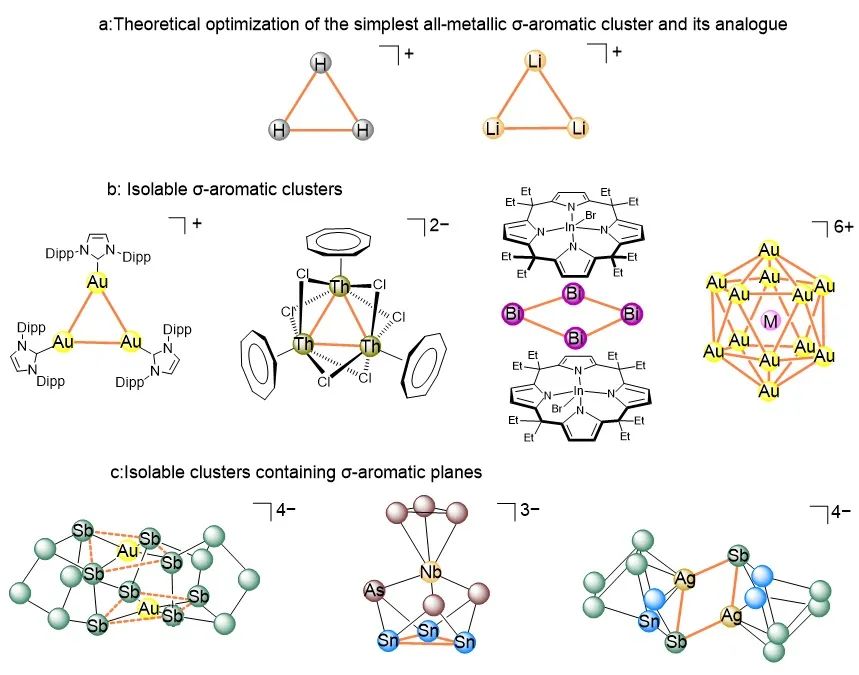
Since the discovery of benzene, the concept of aromaticity has profoundly advanced our understanding of the electronic structure and stability of compounds. Aromaticity has become one of the most significant concepts in chemistry. Over the past few decades, this concept has expanded beyond the original π-electron delocalization system to include other types of electrons, such as σ-electrons. σ-Aromaticity was first introduced to explain the anomalous magnetic properties of cyclopropane. Aromaticity has also transcended the realm of planar organic compounds, extending to inorganic compounds with diverse structures, thereby opening the door to new forms of aromaticity, including 3D aromaticity. Substantial efforts have been devoted to predicting aromaticity through theoretical calculations. A notable example is Li3+, an H3+ analog with two σ-electrons delocalized over the simplest all-metal cluster, although there is less consensus about its σ-aromaticity. The limits of theoretical models pose significant obstacles to the accurate prediction and ultimate synthesis of all-metal aromatic clusters, particularly with regard to the need to consider electron correlation, as well as relativistic and environmental effects in the calculations. Typical isolable clusters include onion-like [E@M12@E20]n− (E = As, M = Ni, n = 3; E = Sb, M = Pd, n = 3, 4; E = Sn, M = Cu, n = 12; E = Bi, M = Pb, n = 6), and the all-metal fullerene [K@Au12Sb20]5–, which exhibit 3D aromaticity. Despite these advances, the synthesis of all-metal aromatic compounds remains in its infancy. Most known all-metal aromatic compounds are limited to small systems, often requiring external stabilization, such as the σ-aromatic [Au3]+ in [(NHCDippAu)3]+, [Zn3]+ in [Zn3Cp*3]+, and [Bi4]4+ in [(Bi4)(EtCxInBr)2]. From a theoretical point of view, [Cu3]+ is also considered a σ-aromatic cluster. Recently, a Th3 cluster has been reported, which shows σ-aromaticity for actinides, despite doubts raised by Foroutan-Nejad and Szczepanik. The relatively larger clusters, [M@Au12]6+ (M = Pd, Pt), found within the ligand-protected [MAu24(SR)18] clusters also exhibit σ-aromaticity and adopt an icosahedral structure. Additionally, although several large aromatic clusters have been reported, their aromaticity originates from smaller aromatic building blocks. Some of these clusters incorporate a σ-aromatic plane, such as [AuSb4] in [Au2Sb16]4–, [Sn3] in [As3Nb(As3Sn3)]3–, and [M2Sb2] (M = Cu, Ag) in [(MSn2Sb5)2]4–. Furthermore, clusters such as [Ge24]4–, {(Ge9)2[η6-Ge(PdPPh3)3]}4–, and [Sn36]8–, feature multiple local σ-aromatic E9 (E = Ge, Sn) fragments.
Recently, Zhong-Ming Sun’s group have successfully isolated and structurally characterized the first Ge/Sn-based trimer, [Co3@Ge6Sn18]5– (1a), formed by the fusion of three [Co@Ge3Sn6] units through a [Ge3] face. Notably, 1a is distinguished by a [Co3Ge3] unit featuring a benzene-like ring with six exceptionally short sides. Theoretical calculations revealed that 1a can be described as a 2e σ-bonded trimer, representing a giant σ-aromatic counterpart to the triatomic σ-aromatic H3+ and Li3+, thus extending the analogy between prototypical molecules and cluster-based aggregates. Relevant achievements were published in J. Am. Chem. Soc., 2025. DOI: 10.1021/jacs.4c16401