In the past 10 years, photoredox catalysis has become a widely accepted, powerful tool for organic synthesis. With the development of transition-metal/photoredox dual catalysis, many unprecedented, previously infeasible cross-coupling reactions have become possible. Metallaphotocatalysis, which takes advantage of the synergistic effects of combining photocatalysis and transition-metal catalysis, has strongly influenced the development of organic chemistry. It is worth noting that photocatalysts can generate free radicals and regulate the oxidation state of metal catalysts, abilities that have greatly expanded the range of electrophilic and nucleophilic reagents that can be used in cross-coupling reactions. Early in the development of photocatalysts, they were used for single electron transfer processes involving substrates containing redox agents, ensuring critical mesolytic fragmentation to provide shell-opening intermediates. Then, the free radical can be intercepted by a transition-metal catalyst and coupled with a suitable electrophilic reagent to form a C(sp3)–C(sp2) bond. In the past few years, chemists have devoted attention to olefin bifunctionalization through metal/photoredox dual catalysis and have developed many methods for intermolecular carboarylation of olefins. Although excellent methods for transition-metal/photoredox synergistic catalysis have been developed, the need for additional free-radical precursors and methods for their activation remains a driving force for research in this field.
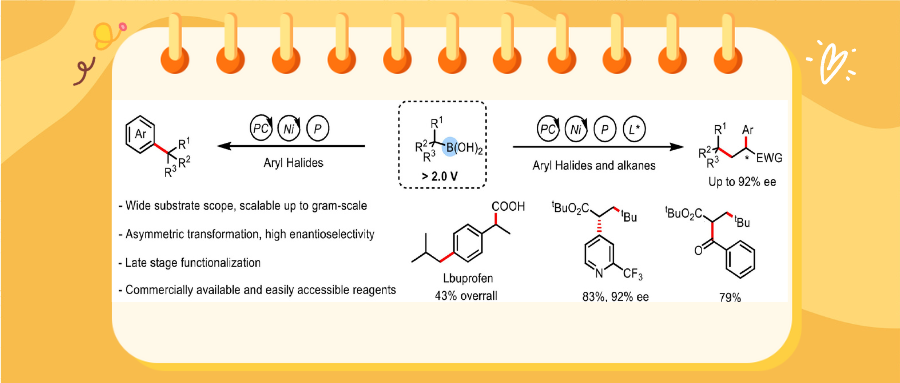
Recently, Qingmin Wang’s group have developed a method for deboronative cross-coupling reactions of alkyl boronic acids by means of photoredox/nickel dual catalysis. We reduced the oxidation potential of the alkyl boronic acids by generating alkyl boronic acid–K3PO4 complexes; this obviated the need for additional reaction components, high reaction temperatures, and expensive catalysts, which limit the utility of previously reported approaches. The method can synthesize compounds with quaternary carbon centers, and when introducing a chiral ligand, it was able to synthesize chiral α-aryl carbonyl compounds. The method demonstrated strong functional group tolerance, and the fact that the synthesis of the alkyl boronic acid substrates is straightforward makes our method ideal for functionalizing biologically relevant alkyl substrates for medicinal chemistry and total synthesis applications. Relevant achievements were published in Chinese Chem. Lett., 2025. DOI: 10.1016/j.cclet.2025.111053.