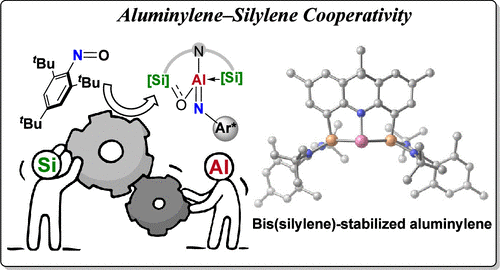
Bimetallic cooperation is widely observed in metalloenzymes and metal catalysts, providing substantial advantages in bond activation and catalysis. In addition to the exceptional reactivities enabled by bimetallic transition-metal complexes, harnessing the cooperative effects of main-group elements has emerged as a promising strategy for fine-tuning the reactivity of main-group compounds. This approach facilitates novel chemical transformations and reactivity patterns that are not achievable with traditional mononuclear entities. As notable examples, frustrated Lewis pairs (FLPs), multiple-bonded main-group compounds, and bimetallic complexes of alkali and alkaline earth metals demonstrate their capabilities as reactive reagents for cooperatively activating strong bonds. Cooperative main-group compounds featuring the most abundant aluminum and silicon elements are attractive, but have been relatively overlooked. Aluminylene and silylene, featuring one lone pair of electrons, have attracted considerable interest as a result of their intriguing electronic structures. While numerous Al(I) and Si(II) species have been isolated, the exploration of silylene-ligated aluminylene remains nearly unexplored. Significantly, the cooperation of two low-valent main-group reaction sites offers a unique opportunity for the multielectron reduction of small molecules.
Recently, Zhenbo Mo’s group have reported the synthesis and characterization of stable bis(silylene)-stabilized aluminylene. Experimental data and DFT calculations show that the three-coordinated Al(I) center features one lone pair of electrons that is delocalized to the formally vacant orbital of Si(II) centers. The cooperation between the proximal aluminylene and silylene enables the multielectron reduction of nitrosoarenes, yielding aluminum imide complex and tetracyclic oxazasilaalanes. Aluminylene serves as an efficient precatalyst for the reductive coupling of nitrosoarenes, facilitating the formation of azoxyarenes. Additionally, this cooperativity aids in the dearomatization of 2-methylquinoline. These findings provide insight into the operation and design of cooperative main-group species that utilize abundant elements of aluminum and silicon. Relevant achievements were published in J. Am. Chem. Soc., 2024. DOI: 10.1021/jacs.4c10323.