As a crucial reactive intermediate with electrophilic properties, difluorocarbene (DFC) represents one of the most frequently employed intermediates in the preparation of fluorinated organic compounds containing a difluoromethylene moiety, which have found widespread applications in the fields of pharmaceutical and agrochemical industry, as well as material chemistry. Due to its high reactivity, DFC must be generated from difluorocarbene precursors under specific conditions. In general, there are two distinct pathways for generating difluorocarbene from X-CF2-Y type precursors. The first pathway involves the use of a nucleophilic species to attack the substituent X to form anionic intermediate Int-I. The subsequent spontaneous departure of substituent Y in the form of an anion generates DFC. Alternatively, DFC could be generated via a photoinduced electron transfer process. In this approach, a photocatalyst is initially excited upon irradiation with light. Single electron transfer (SET) from the excited state of photocatalyst to the DFC precursor, coupled with the leaving of the anion X–, results in the formation of the radical intermediate Int-II. Subsequently, the leaving of substituent Y in a neutral form generated DFC, along with the electron transfer back to the oxidized state PC+ to reform the PC. From gaseous E-CF2-E precursors, DFC is also generated primarily through two distinct pathways. In the first pathway, two electrons are transferred to the gaseous CF2Br2 reagent via electrolysis or from a reductant, resulting in the formation of DFC and simultaneously releasing the bromide ions. Alternatively, the second pathway involves the irradiation of gaseous CF2N2 with an ultraviolet lamp or ultraviolet laser for spin-allowed excitation (SAE) to generate DFC and dinitrogen. However, the aforementioned pathways typically require initiators, additives, and catalysts or require relatively intense reaction conditions to generate DFCs.
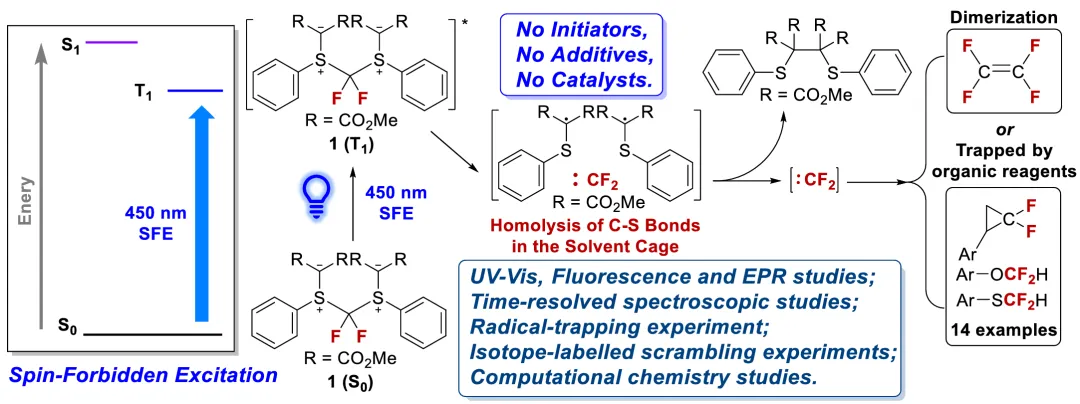
Recently, Tianfei Liu’s group have synthesized a new DFC precursor called difluoromethane bis(sulfonium ylide) with a symmetrical structure. Based on the identification of the product, the results of reagent 1’s UV–vis and fluorescence spectra, and the theoretical analysis on its spin–orbit coupling constants, we conclude that this DFC precursor 1 can undergo spin-forbidden excitation under 450 nm blue light irradiation to release DFC, and the reaction condition does not require the addition of any additives or catalysts. The generated DFC intermediate can dimerize to produce tetrafluoroethylene under such conditions without substrates, and it can also achieve difluorocyclopropanation of substrates in the presence of styrene derivatives or difluoromethylation of X–H bonds (X = O, S) in the presence of phenol and thiophenol derivatives. This new difluorocarbene precursor 1 may provide an improved method for synthesizing organic compounds containing difluoromethylene groups. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c10939.