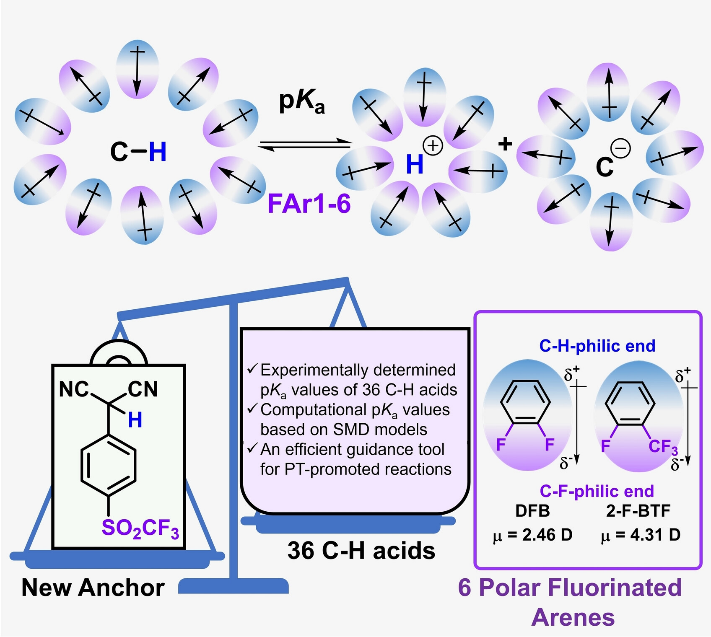
A C-H bond’s pKa value is an important physicochemical parameter defining the free energy of proton dissociation from this C-H bond in an organic compound. Additionally, it provides a quantitative basis for the rational design and development of functionalization reactions to break and form C-H bonds. To meet the increasing demand for accurate testing and prediction of pKa values of C-H bonds in chemistry, material science and life sciences, experimental determination of such pKa values have been completed in aqueous solution and various common organic solvents, where the majority of these solvents are excellent media for organic reactions. During the past few years, prediction methods based on experimentally determined pKa data in aqueous solutions and organic solvents have also been vigorous developed. As of yet, no experimental measurement or theoretical investigation has been conducted on the pKa values of C-H bonds in fluorous solvents, which makes it difficult for people to rationally comprehend how C-H bonds break and reform in such solvents. Fluorous solvents, however, play an important role in organic synthesis due to their unique physical and chemical properties that are different from those of water and traditional organic solvents. In the past four decades, a number of special reactions have been developed that take advantage of the fact that some highly fluorinated solvents can form phase interfaces with water and organic hydrocarbon solvents at room temperature. Fluorinated biphasic systems, developed by using fluorous solvents as reaction media, have revolutionized synthetic organic, catalytic and biocatalytic processes from the perspective of green chemistry. Additionally, they have become valuable tools for a wide range of medical applications, ranging from blood substitutes to organ preservation to liquid ventilation to cancer therapy, saving the lives of patients through a series of medical miracles. Fluorous solvents have been widely used in the above-mentioned fields due to their special physicochemical properties. Nevertheless, the solubility of traditional pKa testing reagents and common organic solutes in fluorous solvents may differ greatly compared to common organic solvents, making it difficult to accurately determine the pKa value of the C-H bond of organic compounds in fluorous solvents through experiments. Furthermore, the lack of basic physicochemical parameters for most of the fluorous solvents has also hampered the construction of their corresponding SMD solvent models, limiting the accuracy of predicting the pKa values of C-H bonds using computational chemistry. The lack of experimentally determined data also makes it difficult to accurately predict the pKa values of C-H bonds in flourous solvents through machine learning or other artificial intelligence methods. It is therefore of great significance to determine the pKa values of C-H bonds in flourous solvents.
Recently, Tianfei Liu’s group have synthesized a new CF3SO2-substituted anchor compound designed for matching the physicochemical properties of polar fluorinated arenes. Its self-dissociation constants in these solvents are used as bases for experimentally determining the pKa values of 36 C-H compounds in them. These experimentally determined pKa values exhibit excellent linear free-energy relationships and correlate well with their corresponding DFT-calculated values. These data indicate that the polar fluorinated arenes are thermodynamically more favorable for deprotonation of ketone derivatives than acetonitrile as reaction media, resulting in enhanced deprotonation-promoted CO2 fixation. The pKa values determined in this work can be used as an important guidance tool for reactions involving the formation and cleavage of C-H bonds in polar fluorinated arenes. Relevant achievements were published in ChemSusChem, 2024. DOI: 10.1002/cssc.202402041.