Cyclobutenones are versatile synthetic building blocks because of the inherent ring strain with the active C═O bond. They undergo unique ring-opening reactions giving complex polycyclic organic molecules. Similarly, silicon analogues of cyclobutenone with an exocyclic Si═O double bond should be of great interest. However, they have remained unknown because of the highly reactive Si═O double bond and the lack of suitable synthetic protocols.
Three-coordinate silanones (A) with the highly polarized Siδ+–Oδ− bond have long been considered as unstable species because of their high tendency to polymerize. Recent studies showed that silanones may be stabilized by a Lewis base (B) or a base and an acid (C). However, the synthesis of silanone A still met with difficulties. Nevertheless, the Fillipou group achieved the first synthesis of a metallo-silanone with a trigonal planar silicon atom. Additionally, by the employment of a bulky amino substituent and the unsaturated ring system, Kato et al. isolated a stable donor-free silanone D. Meanwhile, the Inoue group reported the imine-stabilized acyclic silanone E. In 2019, the Iwamoto group reported the kinetically stabilized dialkyl-substituted silanone F. Despite these achievements, stable three-coordinate silanones are still scarce. However, the half-life of these known silanones could only reach a maximum of 4 d, and the shortest one is only 0.5 h. Therefore, the synthesis of stable three-coordinate silanones is still a great challenge.
Recently, Chunming Cui’s group have isolated the first strained three-coordinate silanone, 2, which is stable in solution and in the solid state even at 70 °C. DFT calculations revealed that the high thermal stability originates from the π- and σ-electron donating effects of the C═C and Si–Si bonds in the ring. The highly polarized Si═O π bond in 2 facilitates C–H and Si–N bond cleavage. Silanone 2 reacted with an N-heterocyclic carbene to give the first isolable 1,2-disilacyclobutadiene 3. Because of the strained Si–Si bond in 2, it undergoes unique photochemical transformations as indicated by the formation of 7 and cyclic alkenyl silylene 8. The studies on the reactivity of silanone 2 indicated its rich chemistry, which could be further explored. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c11194.
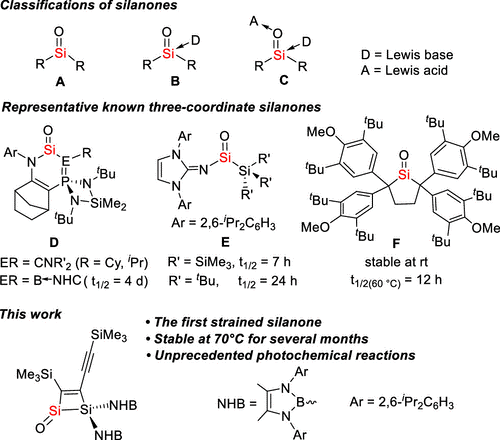
Figure. Previous Work on Silanones and This Work.
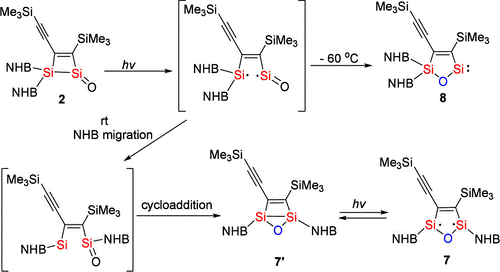
Scheme. Proposed Mechanisms for Photolysis of 2.