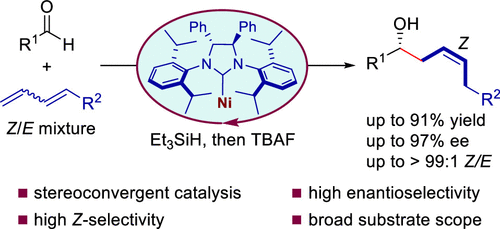
Stereoconvergent reactions have emerged as a powerful strategy for transforming mixed stereoisomers into stereodefined products. These reactions are particularly promising for converting Z/E-mixed olefins, commonly encountered as starting materials in organic synthesis. Traditional olefination processes typically produce these mixtures, yet separating them is challenging due to their different configurations. In asymmetric reactions involving olefins, the outcome often depends on whether the Z or E isomer is used, leading to products with varying absolute configurations. This issue is further exacerbated when chiral catalysts, effective with E olefins, demonstrate reduced efficacy and selectivity toward Z olefins. Despite significant interest, developing effective stereoconvergent and asymmetric methods has proven difficult, particularly in achieving high enantioselectivity and yields for converting Z/E-mixed olefins into chiral molecules.
Recently, Li-Jun Xiao’s group reported a stereoconvergent and highly enantioselective method for synthesizing Z-homoallylic alcohols via the nickel-catalyzed reductive coupling of Z/E-mixed 1,3-dienes with aldehydes. This process is enabled by an N-heterocyclic carbene ligand characterized by C2-symmetric backbone chirality and bulky 2,6-diisopropyl N-aryl substituents. Our method achieves excellent stereocontrol over both enantioselectivity and Z-selectivity in a single step, producing chiral Z-homoallylic alcohols that are valuable in natural products and pharmaceuticals. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c07907.