Silylenes, divalent silicon species, have received a great deal of attention in the past several decades because of their rich chemistry in synthetic chemistry, catalysis, and activation of small molecules. Bis-silylenes, in which two closely located Si (II) centers are bridged by one or more atoms (groups), are of great interest because they may display synergistic effects in chemical transformations. Driess and co-workers recently reported several bis-silylenes of the type LSi-linker-LSi (L = PhC(tBuN)2), in which the two three-coordinate silylenes exhibited unique reactivity in the activation of small molecules and chelating effects for the stabilization of a low-valent main group and transition metal complexes, respectively. However, bis-silylenes with the formula of RSi-E-SiR with acyclic two-coordinate Si (II) atoms bridged by a single atom are still elusive because of the inherent high reactivity of this type of silylenes.
Although Lewis base-stabilized three-coordinate silylenes and N-heterocyclic silylenes have been extensively studied in the past two decades, acyclic two-coordinate silylenes bearing carbon-, silicon-, and boron-based substituents are much less common because of their extremely high reactivity arising from their small HOMO–LUMO gaps. At present, only a couple of acyclic two-coordinate silylenes have been isolated. In recent years, there is growing interest in boryl-substituted low-valent main group species because of their powerful capacities for the activation of small molecules. However, there is only one example of isolable N-heterocyclic boryl-substituted silylene, namely, (HCNAr)2BSi[(NAr)SiMe3)] Si (Chart 1, I, Ar = 2,6-iPr2C6H3) that has been reported.
Recently, Chunming Cui’s group have prepared the first oxo-bridged bis-silepin 2 by the oxidation of disilyne 1 with one equiv of Me3NO. The controlled oxidation of 1 may involve the silylene-silanone intermediate (NHB)Si–Si(O)(NHB) 2′-intA, which then underwent the silylene migration to the oxygen atom to give 2. DFT calculations disclosed that bis-silepin 2 is only more stable by 13.4 kcal/mol than the corresponding oxo-bridged bis-silylene intermediate 2′. Bis-silepin 2 only reacted with one molecule of P4, PhSiH3, Ph2CO, thiophene, and N-heterocyclic carbene IMe4, leading to the sequential oxidative-addition of the two same or different bonds of the one molecule with the formation of novel cyclic or polycyclic silicon systems, indicating the collaborative effects of the two Si centers on the activation of the two bonds. Furthermore, the reaction of 2 with di-m-tolyethyne and DMAP resulted in the initial oxidative-addition of the C≡C triple and aromatic C═N double bonds, followed by the oxidative–addition of one aromatic C═C and C═N bonds in benzene and DMAP with the collaboration of the two silicon centers to give two new bridges. These results demonstrated the distinctive reaction patterns of 2 from monosilylenes, facilitated by the proximity of the two Si centers. The formation of novel polycyclic silicon systems indicated the high potentials of 2 for the development of new silicon chemistry. Further investigation of the reactivity of bis-silepin 2 toward small and organic molecules is still undertaken in our group. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.4c10961.
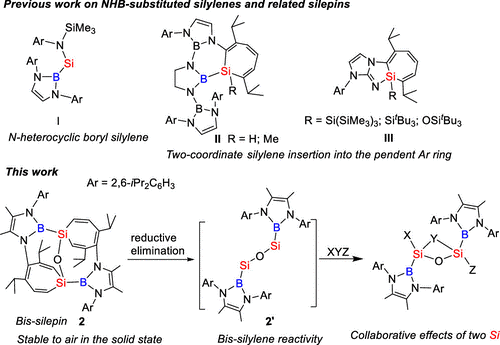
Figure. Comparison of Previous Work on Two-Coordinate Acyclic Silylenes with This Work.
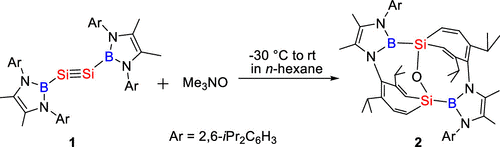
Scheme. Synthesis of Bis-Silepin.