Several marine microorganisms, such as Gymnodinium breve and Gambierdiscus toxicus, produce neurotoxins that can accumulate in fish and shellfish via the food chain. Because these structurally complex neurotoxins are extremely rare in nature, considerable effort has been devoted to isolating them and elucidating their structures. It has been found that these neurotoxins are a class of polycyclic polyethers with a ladder-like structure, also known as marine ladder polyethers (MLPs). The structures of some representative are MLPs—brevetoxins B and A, hemibrevetoxin B, gambieric acid-A, brevenal, ciguatoxin 3C, and maitotoxin. Studies have shown that MLPs exhibited a diverse array of potent biological activities. Neurotoxic MLPs can bind to voltage-gated sodium, potassium, or calcium channels. Worldwide, approximately 20,000 cases of seafood poisoning known as ciguatera are reported annually, and its symptoms are attributed to sodium channel depolarization induced by ciguatoxins (ciguatoxin 3C LD50=0.25–4 μg/kg). However, brevenal, which is not neurotoxic, can competitively bind to voltage-gated sodium channels and act as an antagonist to ciguatoxins and brevetoxins. Brevenal might therefore be useful for the treatment of brevetoxin and ciguatoxin poisoning. Moreover, a 2005 study in cellular and animal models of cystic fibrosis showed that brevenal is therapeutically active at nanomolar to picomolar concentrations. In addition, gambieric acid-A, which is nontoxic, shows antifungal activity that is 2000 times that of amphotericin B. Receptor proteins for brevetoxins and ciguatoxins have been identified, but the biological targets of many other MLPs have not yet been elucidated. MLPs are of great interest to biologists because the study of their bioactivities may lead to the discovery of unknown biological events.
Marine ladder polyethers have attracted the attention of chemists and biologists because of their potent biological activities. Synthetic chemists have attempted to construct their polyether frameworks by epoxide ring-opening cascades, as Nakanishi hypothesis describes. However, Baldwin's rules of ring closure state that exo-selective intramolecular cyclization of epoxy alcohols is preferred over endo-selective cyclization.
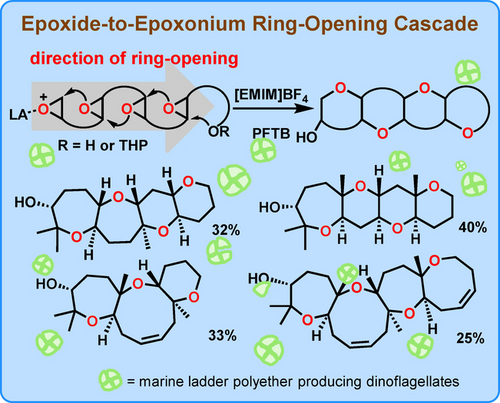
Recently, Jin Qu’s group investigated epoxide ring-opening cascades of polyepoxy alcohols in [EMIM]BF4/PFTB (1-ethyl-3-methylimidazolium tetrafluoroborate /perfluoro-tert-butyl alcohol) and found that all-endo products were formed via epoxide-to-epoxonium ring-opening cyclizations (not restricted by Baldwin's rules, which only apply to intramolecular hydroxyl-to-epoxide cyclizations). They determined that the key factor enabling polyepoxy alcohols to undergo a high proportion of all-endo-selective cyclization was inhibition of exo-selective hydroxyl-to-epoxide cyclization starting from the terminal hydroxyl group of a polyepoxy alcohol. By introducing a slow-release protecting group to the terminal hydroxyl group, they could markedly increase the cyclization yields of polyether fragments with hydrogen atoms at the ring junctions. For the first time, They constructed consecutively fused six-membered-ring and fused seven-, eight-, and nine-membered-ring polyether fragments by epoxide-to-epoxonium ring-opening cyclizations through the addition of a suitable Lewis acid. They also suggest that the biosynthesis of marine ladder polyethers may proceed via epoxide-to-epoxonium ring-opening cyclization of polyepoxide. Relevant achievements were published in Angew. Chem. Int. Ed., 2024, DOI: 10.1002/anie.202403597.