1,2-migration is a fundamental isomerization reaction in organic chemistry. Vinylidene-alkyne isomerization is a typical process, which have attracted significant interest due to its involvement in a series of chemical reactions. Highly accurate calculations have provided deep insight into this isomerization reaction. However, vinylidenes (R2C=C:) can only be generated under extreme conditions and spectroscopically investigated. Over the past decade, heavier analogues of vinylidenes (R2E=E:, E=Si, Ge, Sn) have been isolated and characterized structurally taking advantage of the base-stabilization concept. Recently, Aldridge et al. reported a base-free digermavinylidene utilizing an electron-donating boryl ligand, which was generated by the rearrangement of the transient digermyne isomer via 1,2-boryl migration. Double 1,2-migration involving the migration of two groups from the same atom to the adjacent atom is far less prevalent. This process is implicated in the transition-metal-catalyzed carbene and nitrene insertion reactions and in the formation of metallotetrylenes. Although 1,2-migration is well-known for main-group compounds, double 1,2-migration has been barely studied. Such isomerization is attractive owing to the formation of a highly reactive main-group species with potential for transition-metal free bond breaking and formation.
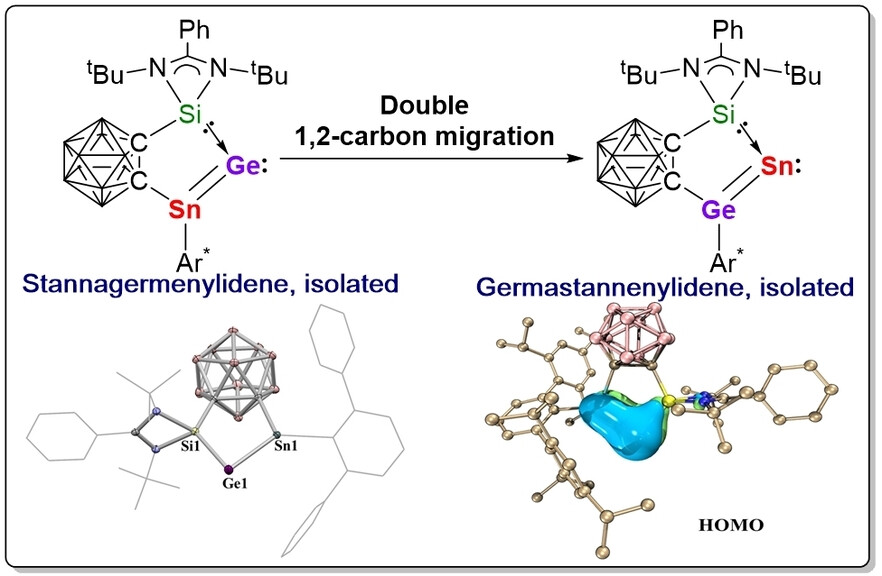
Recently, Zhenbo Mo’s group have established the synthesis and structural characterization of the first stannagermenylidene (3). Compound 3 is thermally unstable and rearranged to a germastannenylidene 4 via unique double 1,2-carbon migration. X-ray diffraction analysis and DFT calculations revealed the presence of strong Sn=Ge double bonds in 3 and 4. Upon treatment with IMe4, 3 undergoes intriguing electron redistribution to give the germylone-stannylene adduct (5). Moreover, 3 readily reacts with (AlCp*)4 to give the heteronuclear aluminyl stannagermyne (6), highlighting its unique reactivity derived from the facile 1,2-carbon migration. This work opens new avenues to pursue double 1,2-migration at two main-group elements. Relevant achievements were published in Angew. Chem. Int. Ed., 2024, DOI: 10.1002/anie.202401570.