Alkylamines constitute a fundamental class of organic compounds with diverse roles in both natural processes and the synthesis of human-made materials. The reactivity of alkylamines is primarily dictated by the nucleophilicity of the nitrogen atom, as exemplified in various N-centered substitution or condensation reactions where N–H bonds of amines are transformed into N–C bonds. Despite their predominant nonredox reactivity, alkylamines also possess distinctive redox capabilities. Of particular significance is the dehydrogenation of alkylamines into imines or iminiums, with the potential to yield aldehydes or ketones upon hydrolysis. This process could open up exciting opportunities for altering alkylamines at their otherwise inert α carbon positions and enable a powerful “crossover” between amine and carbonyl compounds. While nonredox N–H-substitution reactions are well established, harnessing the redox reactivity of alkylamines for the generation of imines has presented substantial challenges for chemists. Mechanistically, the crux of the alkylamine dehydrogenation revolves around the cleavage of the unreactive α C–H bond. Various strategies have been explored to effect this Cα–H bond cleavage, including deprotonation (H+ transfer) with vicinal dicarbonyl reagents, hydrogen atom transfer or single electron transfer/deprotonation, and metal-catalyzed hydride (H–) transfer reactions. In addition to metal-catalyzed pathways, hydride transfer within alkylamines can also occur without metal assistance, although primarily in intramolecular settings. Despite the significant advances in intramolecular reactions, our understanding of amine hydride liberation remains limited.
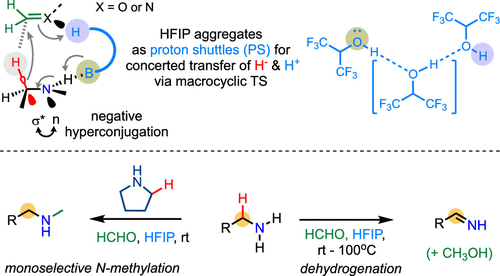
Recently, Gong Chen’s group reported a straightforward approach employing a formaldehyde (HCHO) reagent in hexafluoroisopropanol (HFIP) solvent to harness the hydride-liberation potential of alkylamines, thereby enabling a range of intermolecular hydride transfer reactions with formaldehyde or formaldimines under mild conditions. By leveraging pyrrolidine as an external hydride donor, primary alkylamines can undergo selective N-methylation via the formaldimine intermediates at room temperature (rt). By tuning the reaction conditions to neutral or mildly basic settings, alkylamines can undergo selective dehydrogenation via direct hydride transfer to HCHO, yielding imines that can be further converted to other carbonyl products. In addition to its applicability across small molecule substrates, these reactions offer unique methods for monoselective N-methylation of amino side chains within peptides, or the conversion of lysine’s nucleophilic amino side chain within peptides into an electrophilic acrolein moiety. Our mechanistic investigations uncover that the key to these intermolecular hydride transfer processes lies in the engagement of solvent-mediated macrocyclic transition states. These transition states can accommodate multiple delicate yet vital orbital interactions, facilitating the concerted transfer of both hydride and proton. In particular, the adoption of suitable conformations that foster strong negative hyperconjugation between the lone electron pair of nitrogen (n) and the antibonding orbital (σ*) of the α C–H bond of the amine constitutes a potent activator of the Cα–H bond, promoting hydride liberation. The aggregates of HFIP molecules also play a pivotal role by acting as critical proton shuttles within the macrocyclic transition state. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: jacs.3c12215.