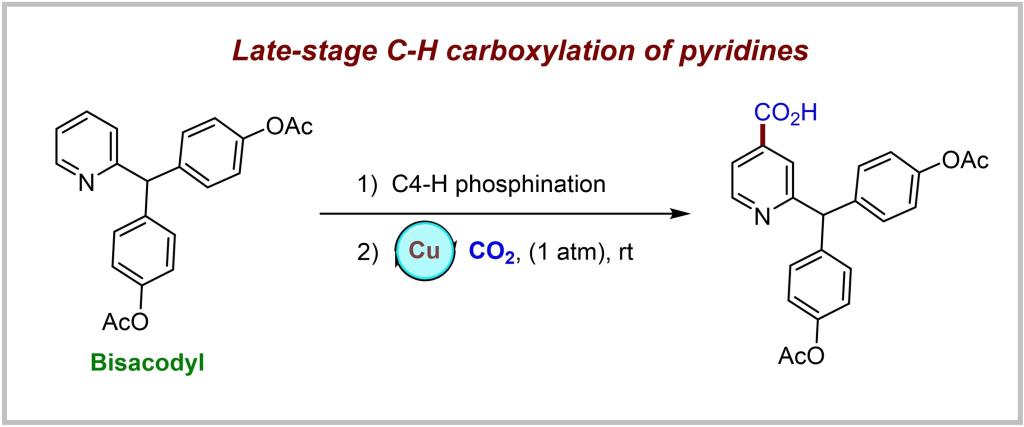
As one of the most favored heterocyclic skeletons, pyridine ubiquitously features in various bioactive compounds, ligands, and functional materials. In this context, developing practical methods for site-selective functionalization of pyridines has aroused distinctive attention. Isonicotinic acid compounds were considered to be prominent pyridine compounds owing to the fact that carboxyl is a core structure of many bioactive molecules. From both economic and green chemistry perspectives, CO2 is an extremely ideal source of carboxyl group. Therefore, it is attractive to install a carboxyl group to the pyridine ring using CO2. However, pyridine is electron-deficient, and CO2 intrinsically has thermodynamic stability and kinetic inertness. Developing a mild and efficient method for selective C−H carboxylation of pyridines with CO2 is a long-standing challenge.
Recently, Baiquan Wang’s group have presented a practical method for the late-stage C4−H carboxylation of pyridines with CO2 via pyridylphosphonium salts. C−P bond carboxylation with CO2 was achieved firstly as a new reductive carboxylation mode. This strategy showed robust reactivity and excellent functional group tolerance, which resulted in the synthesis of diverse valuable isonicotinic acid derivatives. This one-pot two-step reaction represents a novel mode of site-selective carboxylation of pyridines, which may be a useful approach in new drug design and discovery in the future. Relevant achievements were published in Angew. Chem. Int. Ed., 2024, DOI: 10.1002/anie.202318572.