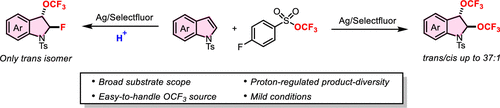
Nitrogen heterocycles are commonly found in natural products and drugs. Dearomatization of indoles to generate indolines has thus received considerable attention. Moreover, dearomatization of indoles using fluorine-containing groups has emerged as an important research topic because fluorine atoms can markedly alter the physicochemical properties and metabolic stability of bioactive molecules. Among the various fluorine-containing groups, the trifluoromethoxy (OCF3) group is featured by the property of strong electron-withdrawing nature and high lipophilicity (πx = 1.04); the introduction of a trifluoromethoxy group into molecules might significantly improve metabolic profiles. For these reasons, the development of methods for the dearomative trifluoromethoxylation of indoles would be desirable.
Recently, Pingping Tang’s group have not only developed the first broadly applicable method for dearomative trifluoromethoxylation of indoles with a wide substrate scope but also accomplished either ditrifluoromethoxylation or fluorinative trifluoromethoxylation by fine-tuning the reaction conditions. Silver difluoride proved to be critical for smoothly achieving the desired transformation. Under optimized conditions, various indoles were converted to ditrifluoromethoxylated indolines in 50–84% yields. Furthermore, OCF3 could act as a leaving group, enabling the synthesis of fluorinated trifluoromethoxylated indolines. Notably, other heteroaromatic rings, and even styrene moieties, also underwent ditrifluoromethoxylation. They anticipate that a diverse array of 3-OCF3 indoline derivatives could be synthesized from ditrifluoromethoxylated indoline products. Relevant achievements were published in J. Am. Chem. Soc., 2024, DOI: 10.1021/jacs.3c11653