Acenes are a unique class of polycyclic aromatic hydrocarbons (PAHs) with linearly fused benzene rings. They have served as high-efficiency luminescent materials (e.g. anthracene) and high-mobility organic semiconductors (e.g. pentacene), thus attracting enormous attention from organic chemists and materials scientists. However, the synthesis of acenes longer than pentacene (the number of fused benzene rings N>5, referred to as large acenes) has remained a huge challenge owing to their high instability under the ambient conditions. Several strategies have been proposed to deal with this problem. For example, the on-surface synthesis under ultrahigh vacuum and the polymer-matrix-assisted synthesis in the solid state have enabled the construction of large acenes up to dodecacene (N=12), and very recently, polyacene has even been achieved by using metal–organic frameworks as the reaction host. In sharp contrast, large acenes synthesized via the traditional wet chemistry have still been limited to nonacene (N=9).
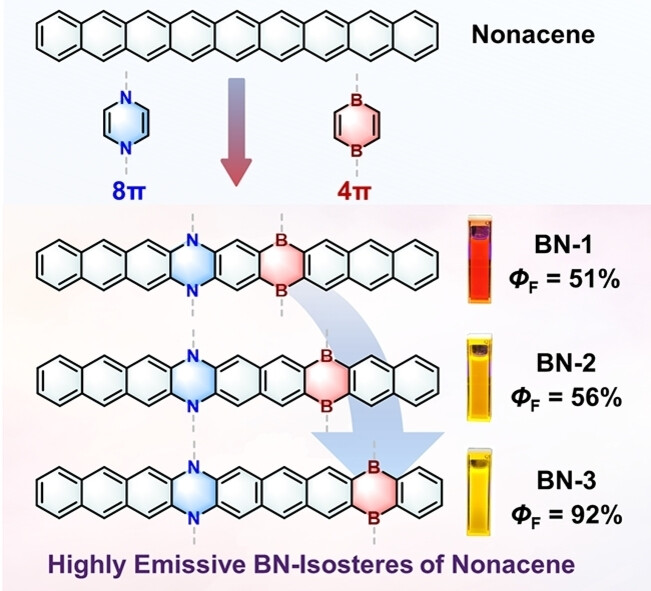
Recently, Xiao-Ye Wang’s group have synthesized the hitherto longest BN-isosteres of nonacene BN-1, BN-2, and BN-3 embedded with a pair of 4π-antiaromatic B2C4 and 8π-antiaromatic N2C4 heterocycles. Photophysical characterizations and theoretical calculations reveal that varying the doping position of the B2C4 and N2C4 rings remarkably affects the contribution of different resonance structures and the local antiaromaticity, which leads to the adjusted molecular orbital arrangement. Consequently, BN-3 exhibits a favorable radiative transition and thus a high ΦF of 92 %. From the synthetic point of view, the nonacene BN-isosteres reported in this work greatly expand the length limit of BN-heteroacenes. Furthermore, the antiaromatic BN-doping mode provides a new design concept to enrich the chemistry of BN-isosteres and to modulate physical properties by molecular orbital engineering, thus offering new opportunities in the future development of BN-embedded conjugated materials. Relevant achievements were published in Angew. Chem. Int. Ed., 2024, DOI: 10.1002/anie.202316596