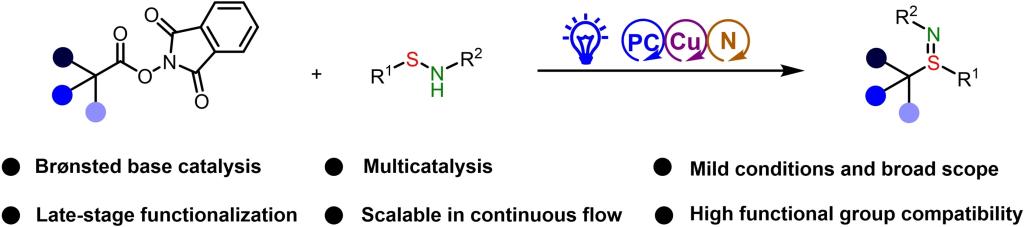
Sulfilimines, the aza-variants of sulfoxides, have attracted considerable attention because of their widespread applications in organic chemistry; they have been reported to serve as nitrene transfer reagents, thioamination reagents, chiral auxiliaries, and directing groups. Moreover, sulfilimine bonds are present in molecules with bactericidal, herbicidal, and pharmacological properties. These bonds have also been discovered in collagen IV: sulfilimine cross-links between methionine-93 and lysine-211 or hydroxylysine-211 stabilize the collagen scaffold. Owing to the stability, biocompatibility, and favorable physicochemical properties of sulfilimines, they have also been used in bioconjugation chemistry. The addition of imidic nitrogen groups is the original driving force to start evaluating this unusual functional group and presents multiple opportunities for manipulating the properties of sulfilimines. For example, modification of the substitution pattern on the nitrogen atom enables modulation of the acidity/basicity and the closely related hydrogen-bond-donating/accepting ability of these compounds. Sulfilimines are also versatile intermediates for the preparation of sulfoximines and sulfondiimines, which are bioisosteres of sulfones and sulfonamides. The need to improve our understanding of the properties of sulfilimines and to increase the commercial availability of sulfilimine building blocks necessitates the development of methods for sulfilimine synthesis. However, methods for radical sulfilimination remain elusive, and as a result, the structural diversity of currently available sulfilimines is limited.
Recently, Qingmin Wang’s group reported the first protocol for decarboxylative radical sulfilimination reactions between sulfenamides and N-hydroxyphthalimide esters of primary, secondary, and tertiary alkyl carboxylic acids, which were achieved via a combination of photoredox, copper, and Brønsted base catalysis. This novel protocol provided a wide variety of sulfilimines, in addition to serving as an efficient route for the synthesis of S-alkyl/S-aryl homocysteine sulfilimines and S-(4-methylphenyl) homocysteine sulfoximine. Moreover, it could be used for late-stage introduction of a sulfilimine group into structurally complex molecules, thereby avoiding the need to preserve labile organosulfur moieties through multistep synthetic sequences. A mechanism involving photocatalytic substrate transformation and copper-mediated C(sp3)-S bond formation is proposed. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202318344
Decarboxylative Ra dical Sulfilimination via Photoredox, Copper, and Brønsted Base Catalysis
Mingjun Zhang, Lixia Liu, Yuhao Tan, Yue Jing, Yuxiu Liu, Ziwen Wang, and Qingmin Wang
Angew. Chem. Int. Ed. , 2023, DOI: 10.1002/anie.202318344