Spin is an intrinsic property of electrons, and studies on electron spin have been at the forefront of materials science and interdisciplinary fields. Spin crossover phenomena are common in open-shell metal complexes and have a wide range of applications in the field of materials science, such as spin-crossover sensors and molecular spintronic materials. In the field of transition metal catalysis, the effect of the catalyst spin state on chemical reactions has also received increasing attention. Studies on the effect of the catalyst spin state are of great value for the development of first row transition metal (3d metal) catalysts. For a long time, precious metal catalysts, especially those based on 4d and 5d metals, have dominated the scientific research and production applications of transition metal catalysis. However, the scarce and non-renewable resources, high prices, and poor biocompatibility of 4d and 5d metals are increasingly becoming factors limiting their applications. Therefore, 3d metal catalysts, especially iron catalysts, have attracted much attention in recent years because of the abundant resources, low prices, and good biocompatibility of their central metals.
Iron catalysts are ideal transition metal catalysts because of the Earth abundant, cheap, biocompatible features of the iron salts. Iron catalysts often have unique open-shell structures that easily undergo spin crossover in chemical transformations, a feature rarely found in noble metal catalysts. Unfortunately, little is known currently about how the open-shell structure and spin crossover affect the reactivity and selectivity of iron catalysts, which makes the development of iron catalysts a low efficient trial-and-error program.
Recently, Shou-Fei Zhu’s group utilized a combination of experiments and theoretical calculations to reveal that the iron-catalyzed hydrosilylation of alkynes is typical spin-crossover catalysis. Deep insight into the electronic structures of a set of well-defined open-shell active formal Fe(0) catalysts revealed that the spin delocalization between the iron center and the 1,10-phenanthroline ligand effectively regulates the iron center’s spin and oxidation state to meet the opposite electrostatic requirements of oxidative addition and reductive elimination, respectively, and the spin crossover is essential for this electron transfer process. The triplet transition state was essential for achieving high regioselectivity through tuning the nonbonding interactions. These findings provide an important reference for understanding the effect of catalyst spin state on reaction. It is inspiring for the development of iron catalysts and other Earth-abundant metal catalysts, especially from the point of view of ligand development. Relevant achievements were published in Nat. Sci. Rev., 2023, DOI: 10.1093/nsr/nwad324.
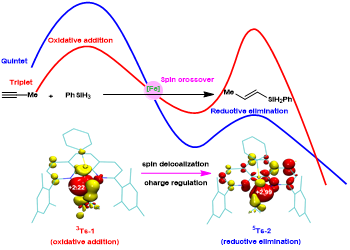
Figure 1. Schematic diagram of spin effect of open shell iron catalyst
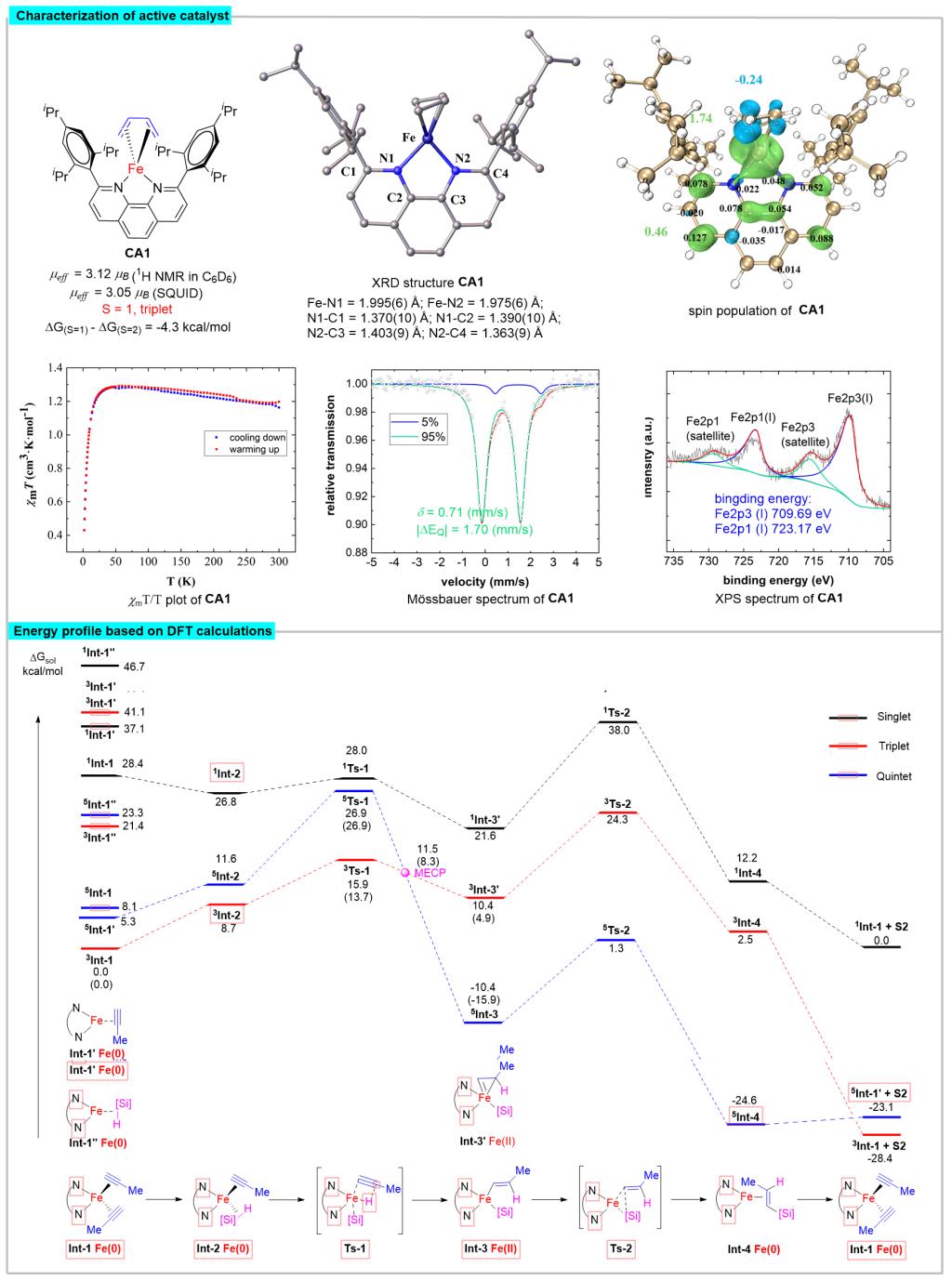
Figure 2. Characterization of active catalysts and DFT calculation of reaction processes
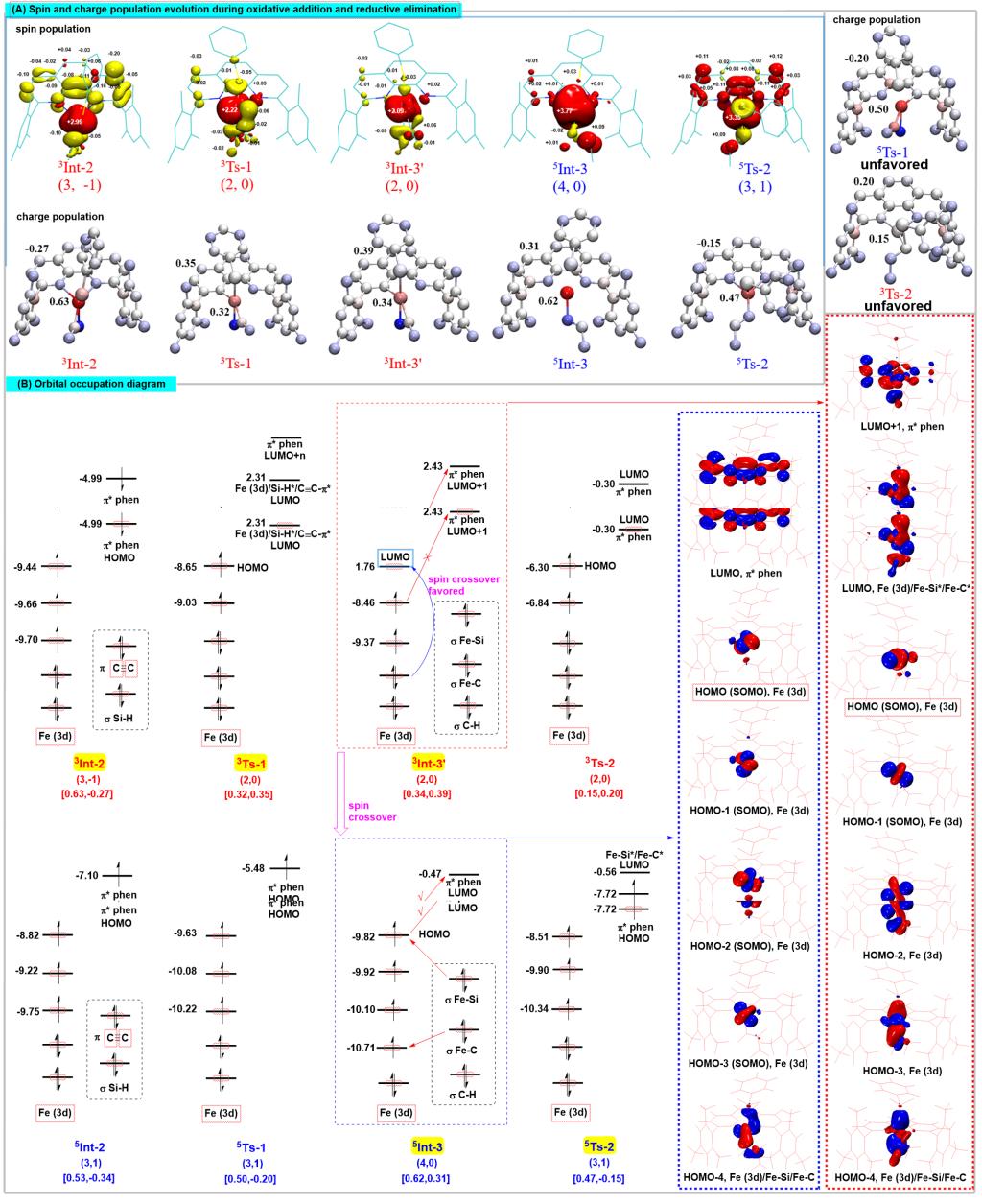
Figure 3. Central charge analysis method
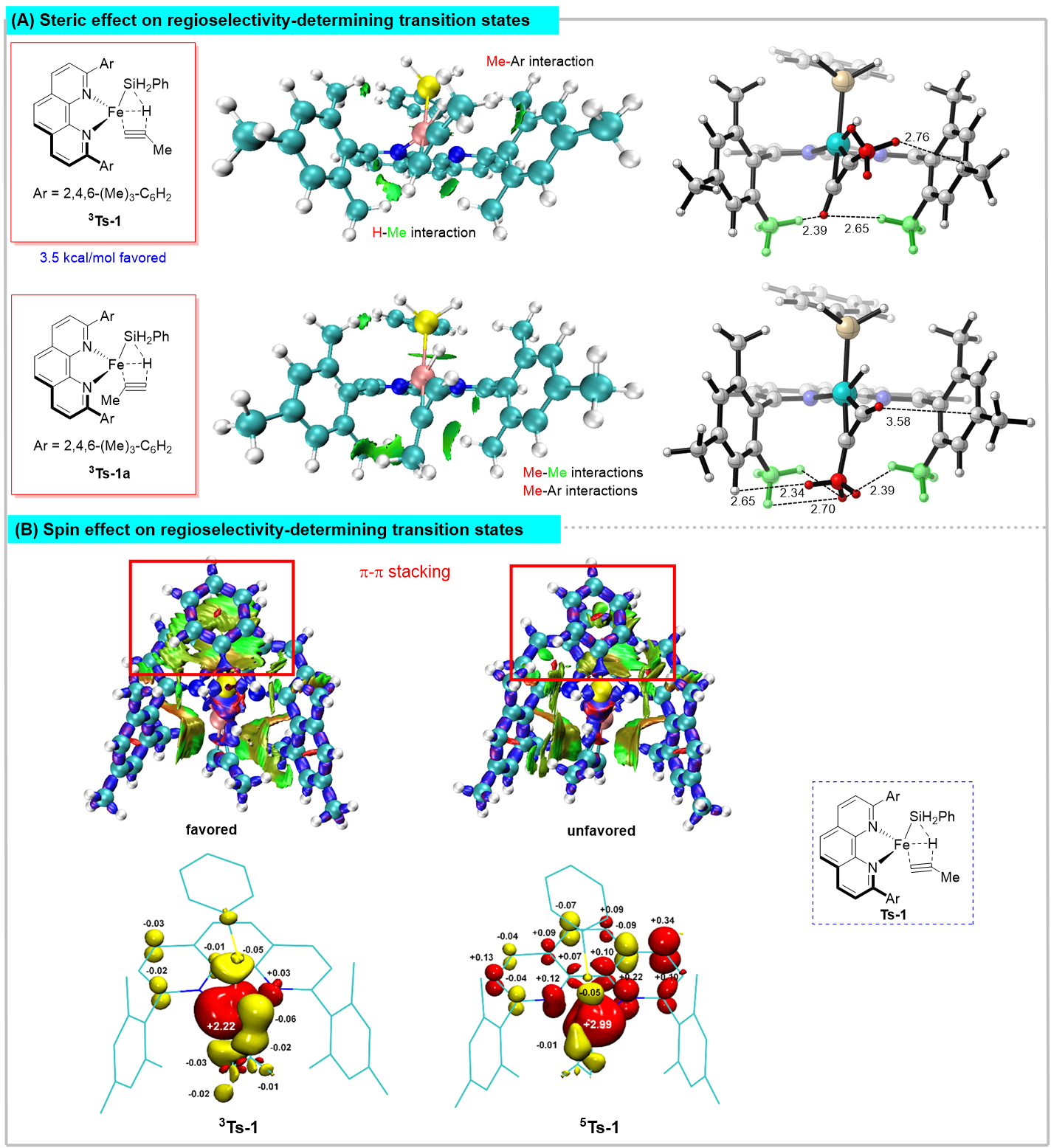
Figure 4. The influence of steric hindrance and spin effect on regional selectivity
Learn More:
Peng He, Meng-Yang Hu, Jin-Hong Li, Tian-Zhang Qiao, Yi-Lin Lu, Shou-Fei Zhu*,
Spin effect on redox acceleration and regioselectivity in Fe-catalyzed alkyne hydrosilylation, Nat. Sci. Rev. DOI: 10.1093/nsr/nwad324.
https://doi.org/10.1093/nsr/nwad324