Intermolecular carbofunctionalization of π-systems via two- or single-electron pathways provides a powerful tool for constructing molecular complexity from readily available starting materials. A classical method is carbofunctionalization of alkynes or alkenes with functionalized reagents, requiring the sacrifice of stoichiometric amounts of molecular fragments even along with stoichiometric oxidants or reductants. Another more attractive alternative is direct insertion of alkynes or alkenes into one chemical bond, because such a process bypasses the loss of molecular fragments and other stoichiometric reagents, offering higher atom and step economy. Owing to this advantage, during the past decades, a broad range of intermolecular carbofunctionalization reactions via direct insertion of alkynes and alkenes into relatively reactive chemical bonds such as C−Cl (Br, I), C−O (S, Se), C−Al and others have been well established. In contrast, direct insertion into relatively inert chemical bonds such as C−C, C−N or C−P bonds are faced with greater challenges, because these bonds are more difficult to activate. Through the introduction of various substrate-activating strategies such as highly-strained small rings, directing groups or polarized chemical bonds, C−C and C−N bonds have also been successfully inserted by alkynes or alkenes, providing a large number of intermolecular dicarbofunctionalization or carboamination reactions. However, in comparison with these advances, the development of intermolecular carbophosphination reaction via direct insertion of alkynes or alkenes into C−P bonds has lagged very behind, which is probably attributed to the following several difficulties: unreactive C−P bonds, not-easily accessible highly-strained P-containing small rings, strong coordination of P atoms to metal catalysts, and difficult C−P bond formation. So far, although numerous cross-coupling reactions that use P-containing substrates as aryl or alkyl coupling reagents have been reported, intermolecular carbophosphination reaction of alkynes or alkenes with C−P bonds still remains an elusive challenge.
Recently, Mengchun Ye’s group developed the first example on Ni−Al bimetal-catalyzed intermolecular carbonphosphination of alkynes with widely-used 5-membered phosphole oxides, providing a series of useful 7-membered phosphepines in up to 94 % yield. Various dialkyl, diaryl and alkylaryl alkynes, together with a wide range of phosphole oxides were well compatible. This Ni−Al bimetallic catalytic system could be applied in a wider range of carbofunctionalization reactions of alkynes or alkenes in future. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202314701.
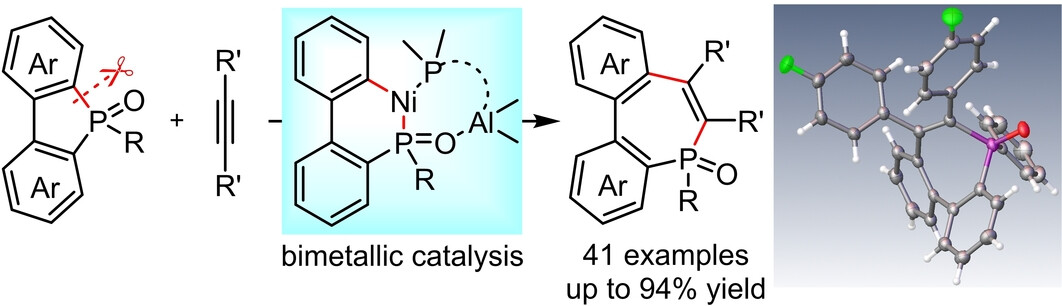
The intermolecular carbophosphination reaction of alkynes or alkenes with unreactive C−P bonds remains an elusive challenge. Herein, we used a Ni−Al bimetallic catalyst to realize an intermolecular carbophosphination reaction of alkynes with 5-membered phosphole oxides, providing a series of 7-membered phosphepines in up to 94 % yield.
Intermolecular Carbophosphination of Alkynes with Phosphole Oxides via Ni–Al Bimetal-Catalyzed C–P Bond Activation
Feng-Ping Zhang, Rong-Hua Wang, Jiang-Fei Li, Hao Chen,*Madala Hari Babu,* Mengchun Ye*
Angewandte Chemie International Edition
DOI: 10.1002/anie.202314701