Trialkylamine groups exist in a wide variety of drugs, naturally occurring alkaloids, and agrochemicals, and their presence is usually essential for the activities of these molecules. Consequently, the development of methods for efficient synthesis of structurally diverse trialkylamines is highly desirable. C−H functionalization is certainly the most efficient and atom-economical approach for generating structural analogs of existing molecules of interest. However, despite the remarkable progress that has been made on amine C−H functionalization reactions over the past decades, methods for C−H functionalization of trialkylamines are underdeveloped. This situation may be due in part to the tendency of electron-rich trialkylamines to decompose in the presence of metal catalysts under oxidative conditions. Another possible explanation is the ineffectiveness of trialkylamines as directing groups for metal-catalyzed C−H activation: the bulk of the amines disfavors metallacycle formation. Thus far, most of the existing studies have been performed on the α−C−H bonds by harnessing their innate high reactivity resulting from the activation by the nitrogen atom. Functionalizing C−H bonds at more distant positions along the alkyl chain remains a formidable challenge. The rare successes include reactions that leverage steric effects to accomplish transition-metal-catalyzed borylation and oxidation reactions at terminal carbons. Moreover, installing a directing group (e.g., an amide) to control the regioselectivity is also a viable strategy. Nevertheless, general strategies for β-selective C−H functionalization of trialkylamines are lacking.
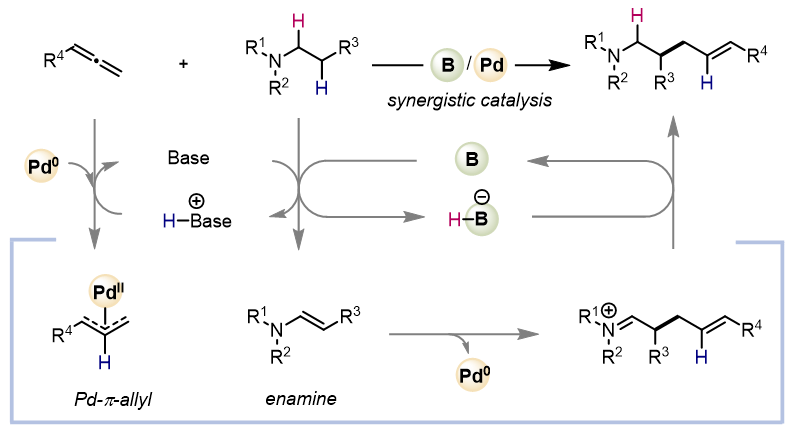
The β-C−H allylation reactions of trialkylamines with allenes were accomplished by a synergistic borane/palladium catalysis. The borane and palladium catalysts promoted the formation of an enamine intermediate from a trialkylamine and a palladium-π-allyl intermediate from an allene, respectively.
β-C–H Allylation of Trialkylamines with Allenes Promoted by Synergistic Borane/Palladium Catalysis
Ming Zhang, Zi-Lu Tang, Heng Luo, Xiao-Chen Wang
Angew. Chem. Int. Ed. , 2023, DOI: 10.1002/anie.202317610