α-Alkylation of carbonyl compounds is one of the most commonly used carbon–carbon bond forming reactions in organic synthesis. In retrosynthetic analyses, α-alkylation of a carbonyl compound is the connection between an enolate of the carbonyl compound and an alkylating agent1. Because most ketones are unsymmetrical (i.e., they have two different alkyl groups), two regioisomeric enolates can be formed upon deprotonation, which can lead to the mixture of structurally isomeric alkylated products, and thus controlling the regioselectivity of α-alkylation of unsymmetrical ketones is a longstanding topic of research in complex molecule syntheses, as documented in almost all organic chemistry textbooks. In the past half a century, remarkable progress has been achieved in the selective alkylation of unsymmetrical ketones at their less-hindered α-sites. However, methods for selective alkylation of unsymmetrical ketones at their more-hindered α-sites remain a challenge. This difficulty escalates when less-substituted enolate is both kinetically and thermodynamically favored over more-substituted enolate. Therefore, it is highly desirable to develop straightforward and general protocols for regioselective alkylation at the more-hinder α-sites of unsymmetrical ketones.
Recently, Li-Jun Xiao & Qi-Lin Zhou’s group reported a nickel-catalysed alkylation of unsymmetrical ketones at the more-hindered α-sites with allylic alcohols. The results indicate that the space-constrained nickel catalyst bearing a bulky biphenyl diphosphine ligand enables the preferential alkylation of the more-substituted enolate over the less-substituted enolate and reverses the conventional regioselectivity of ketone α-alkylation. The reactions proceed under neutral conditions in the absence of additives, and water is the only byproduct. The method has a broad substrate scope and permits late-stage modification of ketone-containing natural products and bioactive compounds. Relevant achievements were published in Nat. Commun., 2023, DOI: 10.1038/s41467-023-38741-w
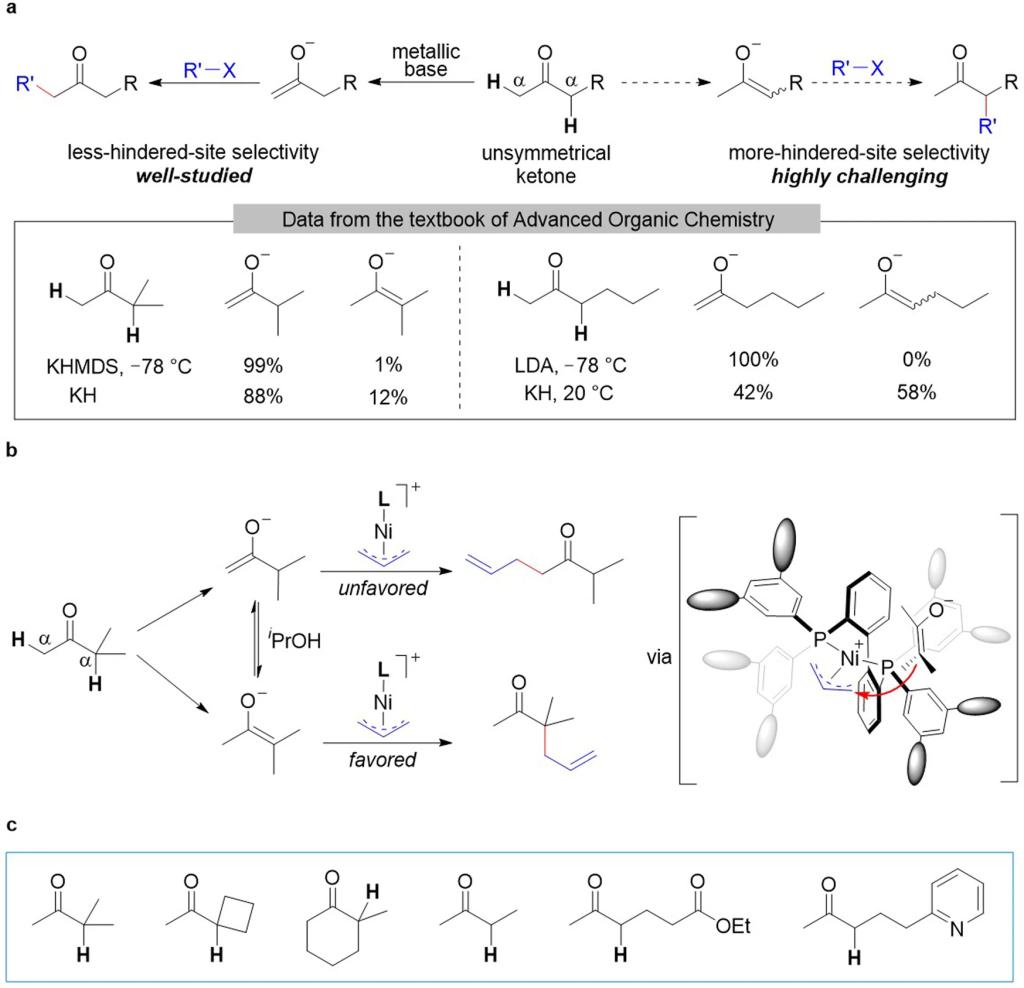
Fig. 1: Regioselective alkylation of unsymmetrical ketones.
a Conventional alkylation of ketones and composition of enolate mixtures generated by various bases. LDA lithium diisopropylamide, KHMDS potassium bis(trimethylsilyl)amide. b Proposed strategy for selective alkylation reaction at the more-hindered α-site. L ligand. c Scope of this allylic alkylation reaction.
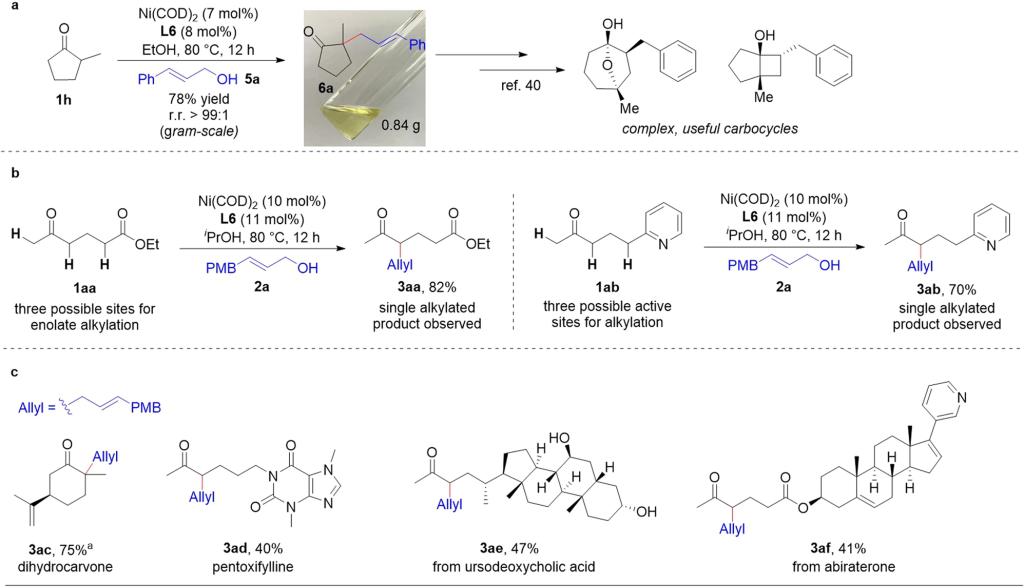
Fig. 2: Applications of nickel-catalyzed allylic alkylation of unsymmetrical ketones.