α, β-Unsaturated ketones are common structures in functional organic molecules and are easy to synthesize. In contrast, robust synthetic methods for β, γ-unsaturated ketones are lacking, despite the fact that these moieties are found in many bioactive molecules and natural products and can be used as building blocks for complex structures. Many of the reported methods are based on disconnection of the bond between the α and β carbons which means α-alkenylation of an enolate or enolate equivalent; whereas disconnection of the bond between the carbonyl group and the α carbon—that is, allylation of an acyl donor—has not been thoroughly explored. In addition, most methods for synthesizing β, γ-unsaturated ketones require a prefunctionalized starting material, which limits the applications of the methods to relatively simple targets. Moreover, these methods suffer from low β, γ regioselectivity. To address these issues, investigators have recently developed a number of mild catalytic reactions. Given that β, γ-unsaturated ketones are privileged scaffolds, their synthesis remains an important challenge, and there is a growing need to blossom new C–H bond activation and late-stage functionalization reactions in an unconventional manner.
Recently, Qingmin Wang’s group reported a mild, operationally simple, multicatalytic method for the synthesis of β, γ-unsaturated ketones via allylic acylation of alkenes. Specifically, the method combines N‑heterocyclic carbene catalysis, hydrogen atom transfer catalysis, and photoredox catalysis for cross-coupling reactions between a wide range of feedstock carboxylic acids and readily available olefins to afford structurally diverse β, γ-unsaturated ketones without olefin transposition. The method could be used to install acyl groups on highly functionalized natural-product-derived compounds with no need for substrate pre-activation, and C–H functionalization proceed with excellent site selectivity. Relevant achievements were published in Nat. Commun., 2023, DOI: 10.1038/s41467-023-38743-8
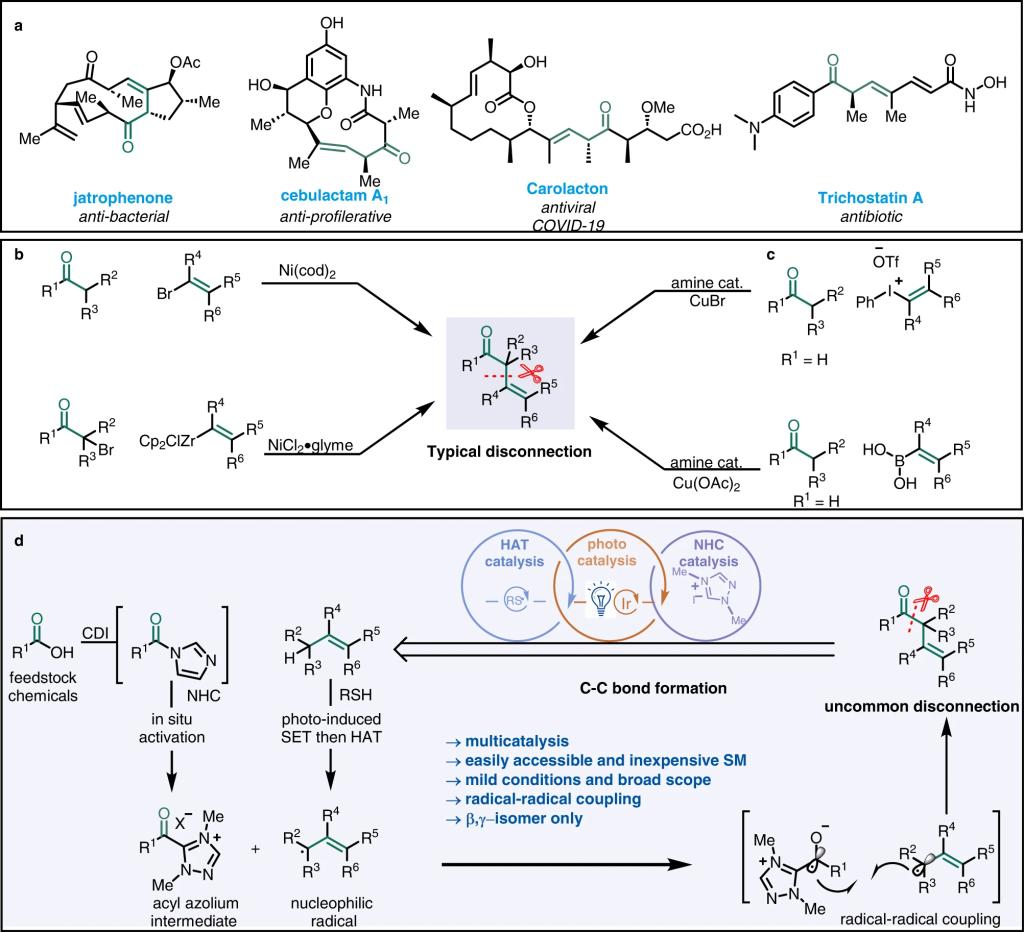
Fig. 1: Bioactive compounds with β, γ-unsaturated ketone motifs and approaches for their synthesis. a Examples of pharmaceutically active agents possessing β, γ-unsaturated ketone motifs. b, c Classic catalytic approaches for β, γ-unsaturated ketone synthesis. d Strategy used in this study.