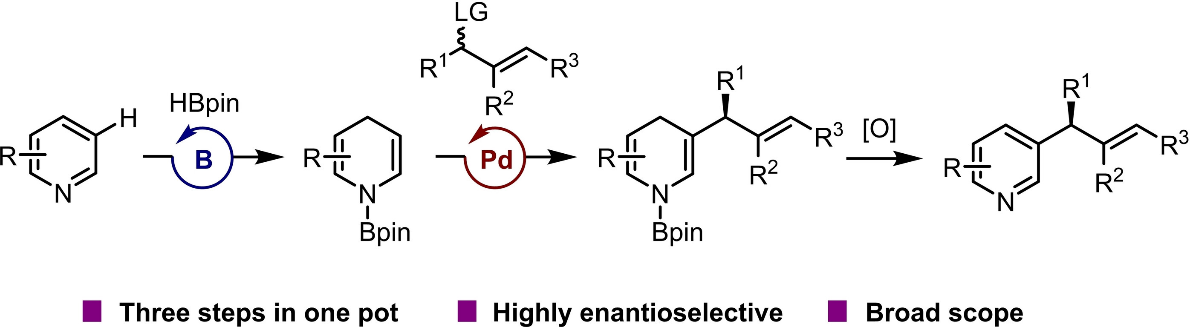
Pyridine rings are prevalent in drugs, agrochemicals, ligands, and functional materials; and many of these compounds possess chiral motifs. Asymmetric C−H functionalization is the most straightforward and atom-economical way to enantioselectively connect a chiral functional group to a pyridine ring. Most of the existing methods involve functionalization of the C−H bond at C2 or C4 via enantioselective addition of radical or anionic nucleophiles or metal-catalyzed C−H activation/enantioselective olefin insertion. As far as C−H functionalization at C3, traditional electrophilic aromatic substitution reactions and metal-catalyzed C−H activation reactions generally give achiral or racemic products. Pd-catalyzed asymmetric allylation reactions constitute a powerful strategy for synthesizing various chiral organic compounds in an enantioselective fashion by reactions of nucleophiles with chiral Pd-allylic complexes generated in situ. And, the Pd-catalyzed ones exhibit complementary regioselectivity (linear with Pd versus branched with Ir) and broader scope (tolerance of multisubstituted olefins) with respect to the allylic electrophile. Therefore, if the Pd-catalyzed allylation reactions are compatible with the tandem process, the structural diversity of C3-allylated pyridines will be significantly improved.
Recently, Xiao-Chen Wang’s group reported a one-pot, three-step method for highly enantioselective C3-allylation reactions of pyridines. The method involved borane-catalyzed dearomative pyridine hydroboration, palladium-catalyzed enantioselective allylation of the dearomatized intermediate, and finally oxidation by air. The method was applicable to a broad range of pyridines, N-heteroarenes, and allylic esters. These findings demonstrate the power of using metal-associated electrophiles with the dearomatization-rearomatization strategy because the choice of a metal and a ligand can markedly influence the regio- and enantioselectivities. Relevant achievements were published in Angew. Chem. Int. Ed., 2023, DOI: 10.1002/anie.202307697