Peptides provide a unique platform for exploring the vast biologically relevant chemical space between small molecules and large biological assemblies. They hold significant potential to address many challenging problems in drug development, such as regulating protein–protein interactions that are difficult to modulate by conventional small-molecule drugs. Like small-molecule drugs and proteins, the three-dimensional structure of peptides is key to their biological activity. While the linear sequence of short ribosomal peptides can be easily assembled in varied sizes and compositions, chemists’ ability to force peptides to adopt stable and diverse secondary structures is quite limited due to the rigidity of backbone amide bonds and the lack of strong and adjustable interactions between the side chains of α-amino acid (AA) residues. Most of the existing strategies for homodetic peptide substrates rely on relatively weak constraining forces to restrict the conformational freedom of peptides, which makes it difficult to modulate the conformations of peptide backbones. Nature has shown that post-translational modifications of ribosomal peptides or nonribosomal synthesis of peptides can generate various unusual structures featuring distinct and diverse backbone conformations. One of the most effective means to generate those structures is to cross-link one or more aromatic side chains in cyclophane-type macrocyclic forms. Because of the rigidity of aryl linkages and the strain of cyclophanes, the cross-linked aromatic side chains can act as braces to control the peptide structure. The strong constraint exerted by the braces can overcome the limitation of conventional conformational control mediated by weak interactions such as H-bonds. However, the cyclophane motifs in those natural products require sophisticated biosynthetic pathways for installation. The synthetic challenges associated with the unusual bond-connecting modes and the ring strain make it difficult to directly employ those linkage motifs in the structural remodeling of regular peptide substrates.
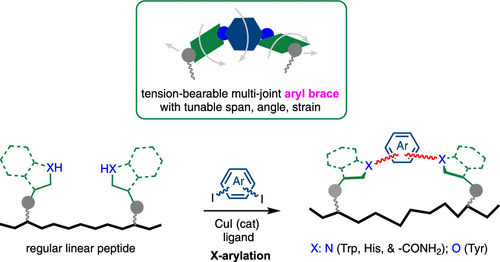
Recently, Gong Chen’s group reported the development of a general and practical strategy to remodel the structural landscape of homodetic peptides by cross-linking two native aromatic side chains with various heteroatom(X)-linked aryl linkers. The aryl linkers can be easily installed via a copper-catalyzed heteroatom-arylation reaction with aryl diiodides. The assemblies of the X-linked aryl units from both side chains and linkers can serve as tension-bearable multi-joint braces to modulate the backbone conformation of peptides in a highly tunable fashion. Relevant achievements were published in J. Am. Chem. Soc., 2023, DOI: 10.1021/jacs.3c03512