The pyridine ring is present in many highly useful organic compounds, including pharmaceuticals, natural products, ligands for metal catalysis, and functional materials. For example, pyridine is the most common heteroarene in small-molecule drugs. In addition, pyridine ligands have numerous applications in metal catalysis because the strong coordination of the nitrogen atom to metals allows for tuning of their electronic properties and also fixes the three-dimensional configuration of metal–ligand complexes. It is noteworthy that many of these pyridine-containing compounds have chiral functional groups attached to the pyridine ring. The established methods for synthesizing such compounds involve reactions of an achiral functional group already on the pyridine ring with a chiral reagent and catalytic asymmetric transformations of prefunctionalized pyridines. However, direct functionalization methods are scarce because the coordinating nitrogen atom of pyridine tends to either deactivate the catalyst or interfere with enantiocontrol.
Recently, Xiao-Chen Wang’s group reported the first examples of Asymmetric intermolecular C–H functionalization of pyridines at C3. Specifically, C3-allylation of pyridines via tandem borane and iridium catalysis. First, borane-catalyzed pyridine hydroboration generates nucleophilic dihydropyridines; then, the dihydropyridine undergoes enantioselective iridium-catalyzed allylation; and finally, oxidative aromatization with air as the oxidant gives the C3-allylated pyridine. This protocol provides direct access to C3-allylated pyridines with excellent enantioselectivity (up to >99% ee) and is suitable for late-stage functionalization of pyridine-containing drugs.
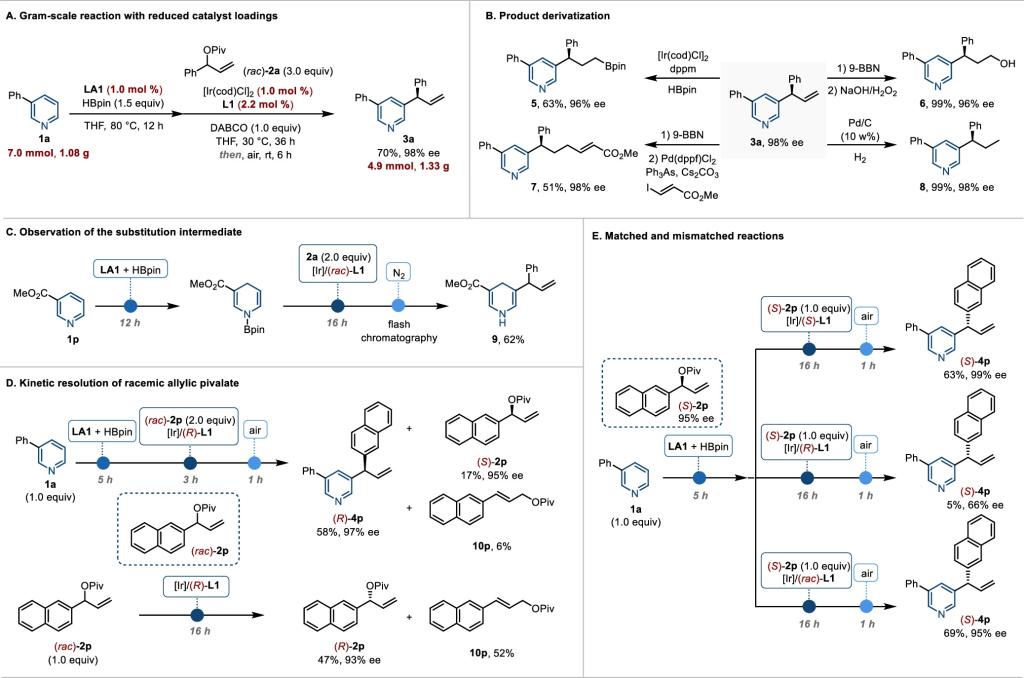
Figure 1. Synthetic applications and mechanistic studies.