Organosilicon compounds are widely used in many fields such as organic chemical synthesis, materials science and medicine due to their special physical and chemical properties. Therefore, it is important to develop simple and efficient methods for the synthesis of organosilicon compounds. In the past decades, transition metal-catalyzed bisilylation reactions between alkynes and bisilanes have been considered as one of the most straightforward methods for the stereoselective synthesis of 1,2-bisilyl olefinic compounds. These 1,2-bisilylated olefins not only possess many unique properties by themselves, but also can be used as important synthetic intermediates for further conversion into many fine chemicals and bioactive molecules containing multi-substituted olefin backbones. Addition of interelement compounds across alkynes is a straightforward strategy for the synthesis of densely functionalized alkenes, which are versatile units in numerous biologically active compounds. Of these processes the bis-silylation of alkynes is of particular interest, due to the synthetic use of the alkenyl silane reaction products. While cis-bis-silylation of alkynes is well known, trans-bis-silyation of alkynes is relatively underdeveloped.
Recently, Dongbing Zhao’s group reported the intermolecular trans-bis-silylation of terminal alkynes, using Pd-catalysis and disilane reagent 8-(2-substituted-1,1,2,2-tetramethyldisilanyl)quinoline (TMDQ), to selectively form trans-bis-silylated alkenes. The reaction process was found to be compatible with aryl and alkyl alkynes as well as those bearing electron-withdrawing groups and other alkenyl or alkynyl functionalities. The use of the reaction was displayed through late-stage functionalization of terminal alkynes bearing natural product or drug motifs and onward synthetic transformations of the trans-bis-silylated alkenes reaction products. Experimental and computational mechanistic studies reveal that the reaction probably proceeds through a combined cis-bis-silylation and Z/E isomerization process and that the use of TMDQ as the limiting reagent is key to the desired reactivity.
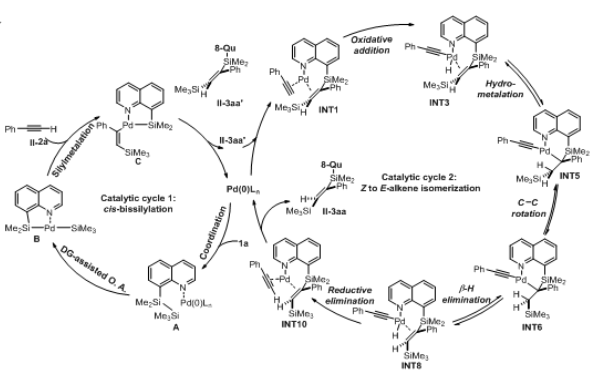
Figure 1. Possible reaction mechanisms and catalytic cycles