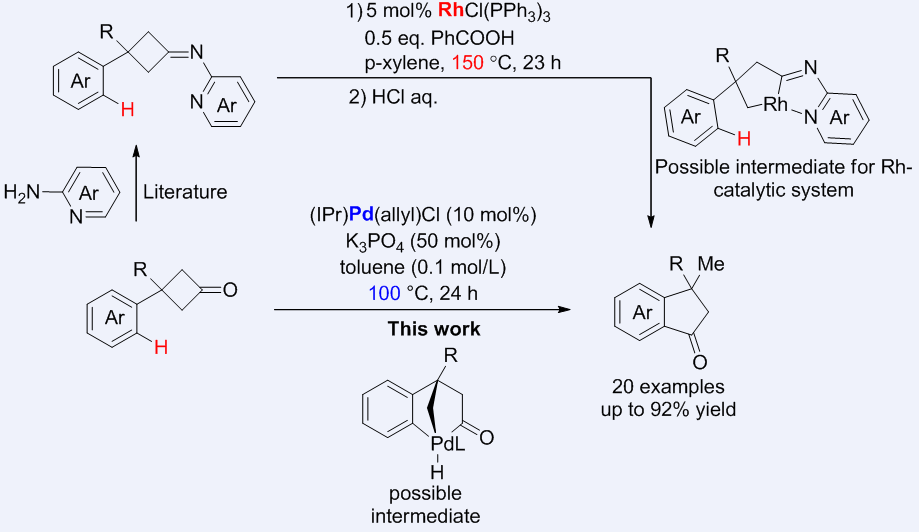
The utilization of cyclobutanonesas the synthon in transition metal catalysis has been made great success. Because C(carbonyl)−C bond of cyclobutanones can be cleaved through strain release. Despite those advancements, the main catalysts in literature are Rh catalysts or Ni catalysts and the reaction with C—H bond is still underdeveloped. Herein, Zhao'group realized the first palladium-catalyzed skeletal reorganization of cyclobutanones involving successive cleavage of C(carbonyl)−C bonds and C—H bond cleavage, which constitutes an rapid access to diverse indanones. In contrast to the previous Rh-catalytic system, the Pd-catalytic system herein involves different mechanism and features several advantages: 1) no need of directing group to facilitate the C(carbonyl)−C bond cleavage; 2) much milder reaction condition and 3) simplified work-up.
Ruirui Li, Xiaonan Shi, and Dongbing Zhao*
Chin. J. Chem. 2023, 41, 1679—1683. DOI: 10.1002/cjoc.202300044