Thiol groups play an indispensable role in both natural and synthetic molecules. Their facile coupling with carbon groups via strong S–C bonds has enabled a myriad of methods for the synthesis and modification of molecules of varied size and complexity. Besides S–C bonds, thiols can also form weaker S–heteroatom bonds such as S–S bond in disulfides and S–N bond in sulfenamides—both of these have unique redox properties and can be selectively cleaved under mild conditions.
Recently, Gong Chen’ group report a method for synthesis of N-acyl sulfenamides via copper-catalyzed nitrene-mediated S-amidation reaction of thiols with dioxazolones. This method is efficient, convenient, and broadly applicable. Moreover, the resulting N-acetyl sulfenamides are highly effective S-sulfenylation reagents for the synthesis of unsymmetrical disulfides under mild conditions. The S-sulfenylation protocol enables facile access to sterically demanding disulfides that are difficult to synthesize by other means. Relevant achievements were published in Nat. Commun., 2022, 13, 6445, DOI: 10.1038/s41467-022-34223-7
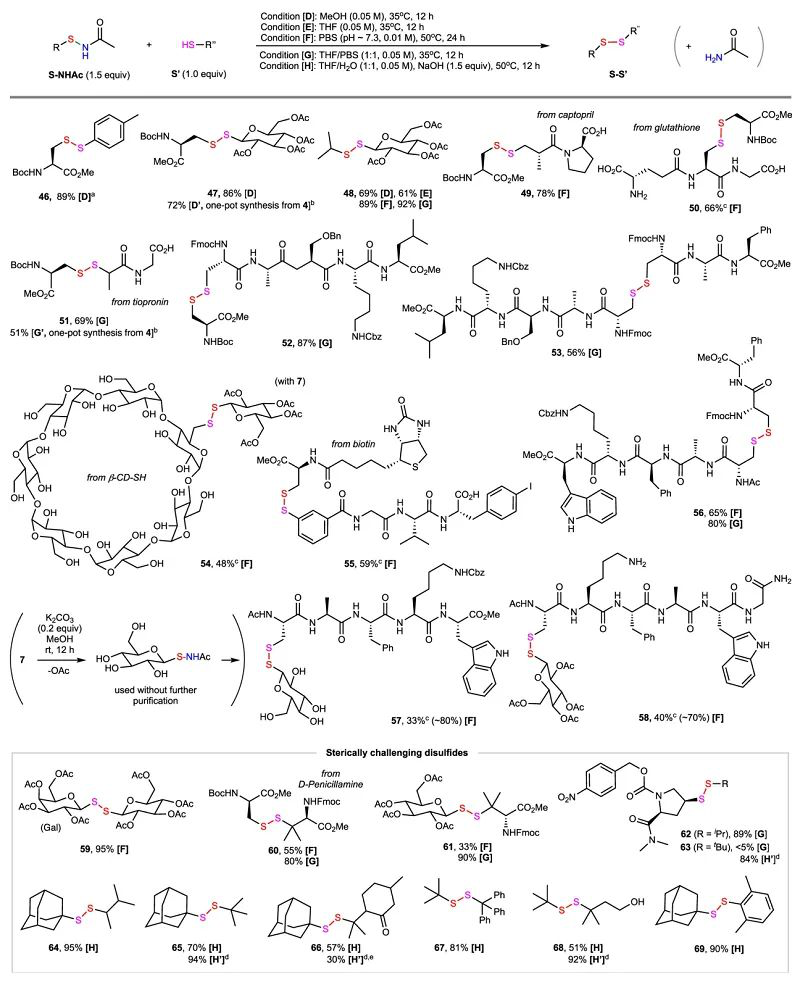
Figure 1. Synthesis and Application of N-Acyl Sulfeneamide in Asymmetric Disulfides