Chiral C2-alkylated pyridines are important structural motifs, widely existing in pharmaceuticals, agrochemicals, and biologically active natural products, as well as catalysts. Compared with either traditional methods often requiring prefunctionalized pyridines and stoichiometric chiral reagents or radical-involved Minisci reactions with the sacrifice of molecular fragments, transition metal-catalyzed C–H alkylation of pyridines with π-unsaturated compounds represents a more attractive alternative owing to better atom and step economy. However, due to the strong coordinative ability of pyridines to metals, which may inhibit the coordination of chiral ligands to metals, the development of enantioselective transition metal-catalyzed C–H alkylation of pyridines has been a formidable challenge, and successful examples are quite scarce.
Recently, Mengchun Ye’ group developed a method in which a chiral phosphine oxide-ligated Ni–Al bimetallic catalyst was used to realize an enantioselective C2–H alkylation of pyridines without the need of a C2-block. A wide range of pyridines, including unsubstituted pyridine, C3, C4, and C2-substituted pyridines, and even complex pyridine-containing bioactive molecules are well compatible with the reaction, providing up to 81% yield and up to 97% ee. The search for wider applications of this bimetallic catalyst is underway in the lab.
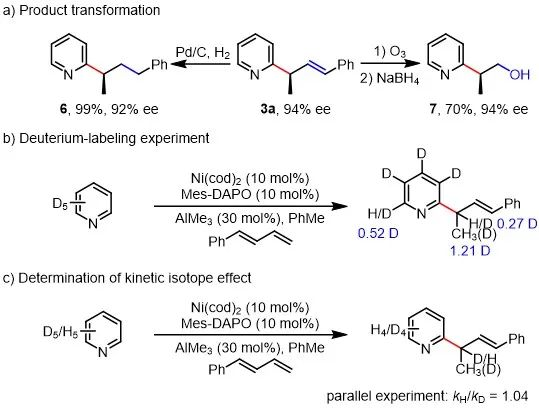
Figure 1. Transformation and mechanism