Allostery, triggered by external stimuli, enables tight regulation of protein functions in the cell environment. In order to mimic nature’s complex allosteric processes, the design and synthesis of well-crafted artificial macrocycles have long been a worthwhile goal in supramolecular chemistry. The concept of photo-controlled molecular encapsulation is generally based on light-responsive guest molecules and very less host macrocycles, because the latter often suffer from tedious synthesis the tendency to hinder the isomerization of light-active species by ring strain or molecular crowding. Thus, construction of such rigid light-responsive macrocycles remain a great obstacle in supramolecular chemistry.
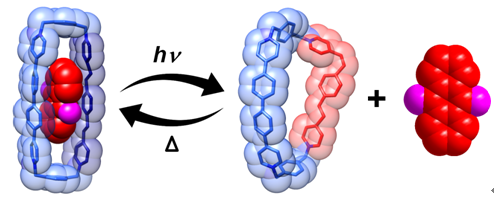
Recently, Prof. Yu Liu and co-workers in collaboration with Prof. J. Fraser Stoddart, designed and synthesized a photo- and thermo-responsive macrocycle that is composed of oligo(p-phenylenevinylene) pyridinium units. Due to the rain strain associated with this molecule, its reversibility is dramatically improved. Under irradiation, the EE-isomer could be isomerized to the EZ-isomer; whereas under thermal conditions the EZ-isomer could be quantitatively converted back to the EE isomer. Because of the allosteric effect of the macrocycle, it is capable of binding or releasing various π-electron-rich and π-electron-deficient aromatics. This photo- and thermo- controlled molecular encapsulation strategy not only provides the incentive to design more advanced photo-responsive host-guest systems and switchable molecular machines but also possesses the potential for applications in the fields of active ingredients stabilization, controllable drug release and toxin separations.
This work has been published on JACS and selected as cover paper.