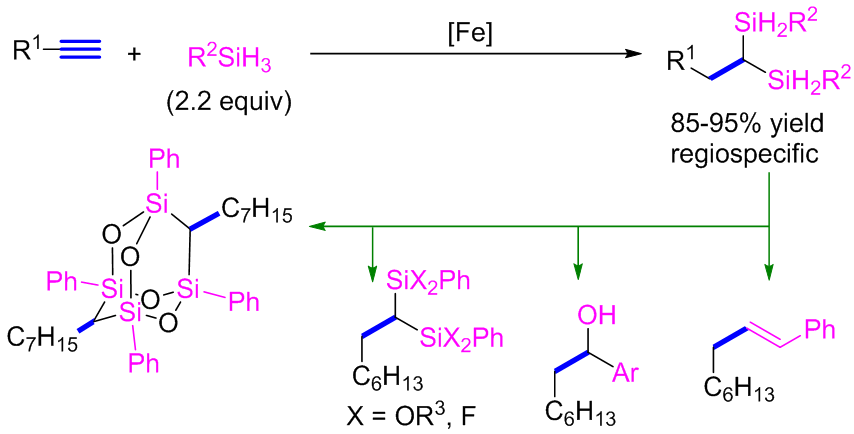
Geminal bis(silanes) are a class of organosilanes with two silicons attached to the same carbon. These compounds are increasingly attractive due to their unique steric and electronic properties, and have been applied as interesting synthons in multiple synthetic reactions. Currently, few methods exist in the literature for the synthesis of geminal bis(silanes), including nucleophilic substitutions, retro [1,4]-Brook rearrangements, and more recently Pd-catalyzed carbene insertion of Si-Si bonds and Cu-catalyzed coupling of C(sp3)-Si with germinal dibromo compounds. These methods often face the issues of narrow substrate scope, low selectivity and poor atom economy. Another serious drawback is that the geminal bis(silanes) produced by these methods are all quaternary silyl groups, which are difficult to derive further, limiting their applications in organic synthesis and material science.
Recently, Prof. Shoufei Zhu and co-workers disclosed the Fe-catalyzed dihydrosilylation of terminal alkynes via their previously developed 2,9-diaryl-1,10-phenanthroline ligand. This reactions allows the synthesis of geminal bis(silane) compounds containing secondary silyl groups. Control experiments show that the reaction proceeds by two sequential Fe-catalyzed hydrosilylations. The iron catalysis first catalyzes the hydrosilylation of the alkyne substrate leading to a vinyl silane intermediate, which then undergoes a second hydrosilylation forming the final products. DFT calculations show that the electronics of the silicon in the vinyl silane intermediate determines the regioselectivity of the second hydrosilylation. The geminal bis(silanes) produced by this methods are all secondary silyl groups. The Si-H bonds in these products are prone to further reactions. These researchers demonstrated several transformations of these geminal bis(silanes), presentation the potential of these compounds in synthetic chemistry and material science. This work expanded the scope of low valent iron catalysis and developed a series of novel valuable organosilanes. This work is currently published on J. Am. Chem. Soc.. The first author of this paper is Mengyang Hu.
This work is supported by the National Natural Science Foundation of China, the Ministry of Education of China, the National Program for Special Support of Eminent Professionals, and the Fundamental Research Funds for the Central Universities.
Meng-Yang Hu, Jie Lian, Wei Sun, Tian-Zhang Qiao, Shou-Fei Zhu*. Iron-catalyzed dihydrosilylation of alkynes: efficient access to geminal bis(silanes),
J. Am. Chem. Soc.
2019
,
141
, 11, 4579.