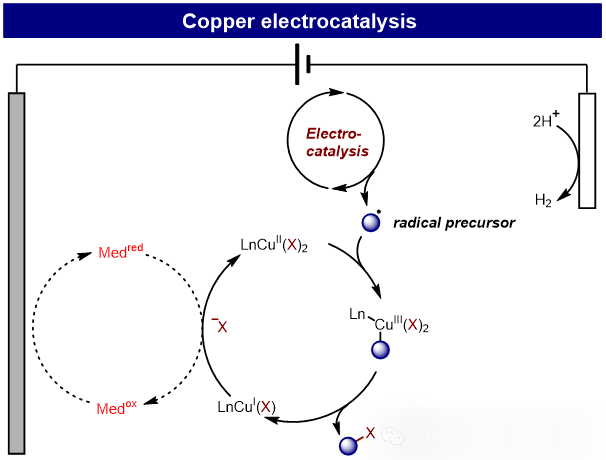
In conventional electrolysis, substrates gain or lose electrons at the cathode or anode, generating highly reactive radical intermediates—making precise control over chemo-, regio-, and enantioselectivity inherently challenging. To address this limitation, integrating electrochemistry with transition metal catalysis has emerged as a promising strategy, leveraging metals’ tunable oxidation states for selective transformations. Upon electron transfer at electrodes, metal catalysts access unique redox states unavailable under traditional chemical conditions.Transition metals offer distinct advantages over conventional mediators: (1) Synergistic Tunability: Electrochemical parameters (potential/current) and transition-metal reactivity are orthogonally adjustable, enabling broad substrate scope. (2) Redox Precision: Ligand engineering allows fine-tuning of metal centers’ redox potentials for controlled activation. (3) Selectivity Governance: Coordination spheres of metal catalysts dictate chemo-, regio-, and stereoselectivity independently of electrode processes. Among these transition metal catalysts, copper-catalyzed electrochemical transformations have attracted considerable attention due to their low cost and high natural abundance, exemplified by reactions such as enantioselective C-H cyanation, decarboxylative cyanation, and olefin difunctionalization. Copper can exist in Cu⁰、Cuᴵ、Cuᴵᴵ and Cuᴵᴵᴵ oxidation states, enabling both single-electron and two-electron transfer pathways. Additionally, the different oxidation states of copper can interact with Lewis acids or form π-coordination with various functional groups. The unique reactivity of this 3d transition metal makes copper a viable alternative to conventional 4d and 5d transition metals (such as palladium, rhodium, and iridium). No additional summary is required, just translate this passage accurately.
Recently, Qing-Min Wang’s group have summarized and introduced the latest advances in electrochemical copper catalysis, with a particular focus on copper-catalyzed electrochemical difunctionalization of alkenes, C−H functionalization, C−C bond activation, and C−X bond activation. Additionally, they discussed potential challenges and future perspectives regarding selectivity control and reaction diversity in organic electrochemical copper catalysis. Relevant achievements were published in Chin. Chem. Lett., 2025. DOI: 10.1016/j.cclet.2025.111673.